|
TABLE 2-5 Major functions of human lipid compounds |
|
|
Function |
Example |
|
Energy |
Lipids
can be stored and broken down later for energy; they yield more energy per
unit of weight than carbohydrates or proteins. |
|
Structure |
Phospholipids
and cholesterol are required components of cell membranes |
|
Vitamins |
Fat-soluble
vitamins: vitamin A forms retinol (necessary for night vision); vitamin D
increases calcium uptake; vitamin E promotes wound healing; and vitamin K is
required for the synthesis of blood clotting proteins |
|
Protection |
Fat
surrounds and protects organs |
|
Insulation |
Fat under the skin
minimizes heat loss; fatty tissue (myelin) covers nerve cells and
electrically insulates them |
|
Regulation |
Steroid hormones regulate many physiological processes.
Examples: estrogen and testosterone are responsible for many of the
differences between females and males; prostaglandins help regulate
inflammation. and tissue repair. |
The Chemical Basis of Life - Lipids - Doctor-dr
June 08, 2022
0
by Microbiology Doctor dr (doctor-dr)(doctor_dr)
Lipids
According to one definition, lipids are organic macromolecules that are insoluble in water. Most of these compounds, many of which have an oily viscosity and a greasy feel, dissolve quickly in nonpolar organic solvents such as ether, alcohol, or benzene, despite being insoluble in water. Lipids, like carbohydrates, are mostly made up of carbon, hydrogen, and oxygen. The percentage of oxygen in lipids, on the other hand, is significantly smaller than in carbs. Other elements, such as nitrogen and phosphorus, are found in many lipids. Lipids are a diverse collection of chemicals that have been categorised in a variety of ways. Triglycerides or fats, phospholipids, steroids, and prostaglandins are all types of lipids.
Lipids are vital biological molecules that serve a variety of functions in the body (Table 2-5). Many are utilised for energy, while others are structurally important and operate as part of cell membranes. Other essential lipid molecules act as vitamins or safeguard key organs by acting as shock-absorbers in certain bodily regions. A specific lipid substance acts as a "insulator material" surrounding nerves, preventing "short circuits" and allowing nerve impulse transmission to be accelerated.
Figure 2 -15 Structural levels of protein. A. Primary structure: determined by number, kind and sequence of amino acids in chain; B: Secondary structure: hydrogen bonds stabilize folds of helical spirals; C: Tertiary structure: globular shape maintained by strong (covalent) intramolecular bonding and by stabilizing hydrogen bonds; D: Quaternary structure: results from bonding between more than one polypeptide unit.
Triglycerides or fats
The most common lipids are triglycerides (triacylglycerols), often known as fats, which serve as the body's most concentrated source of energy. To manufacture or construct a fat molecule, two types of building blocks are required: glycerol and fatty acids. Three fatty acids are linked to each glycerol molecule. Each fat molecule has the same glycerol building component. As a result, the chemical composition of every fat is determined by the exact kind of fatty acid molecule or component.
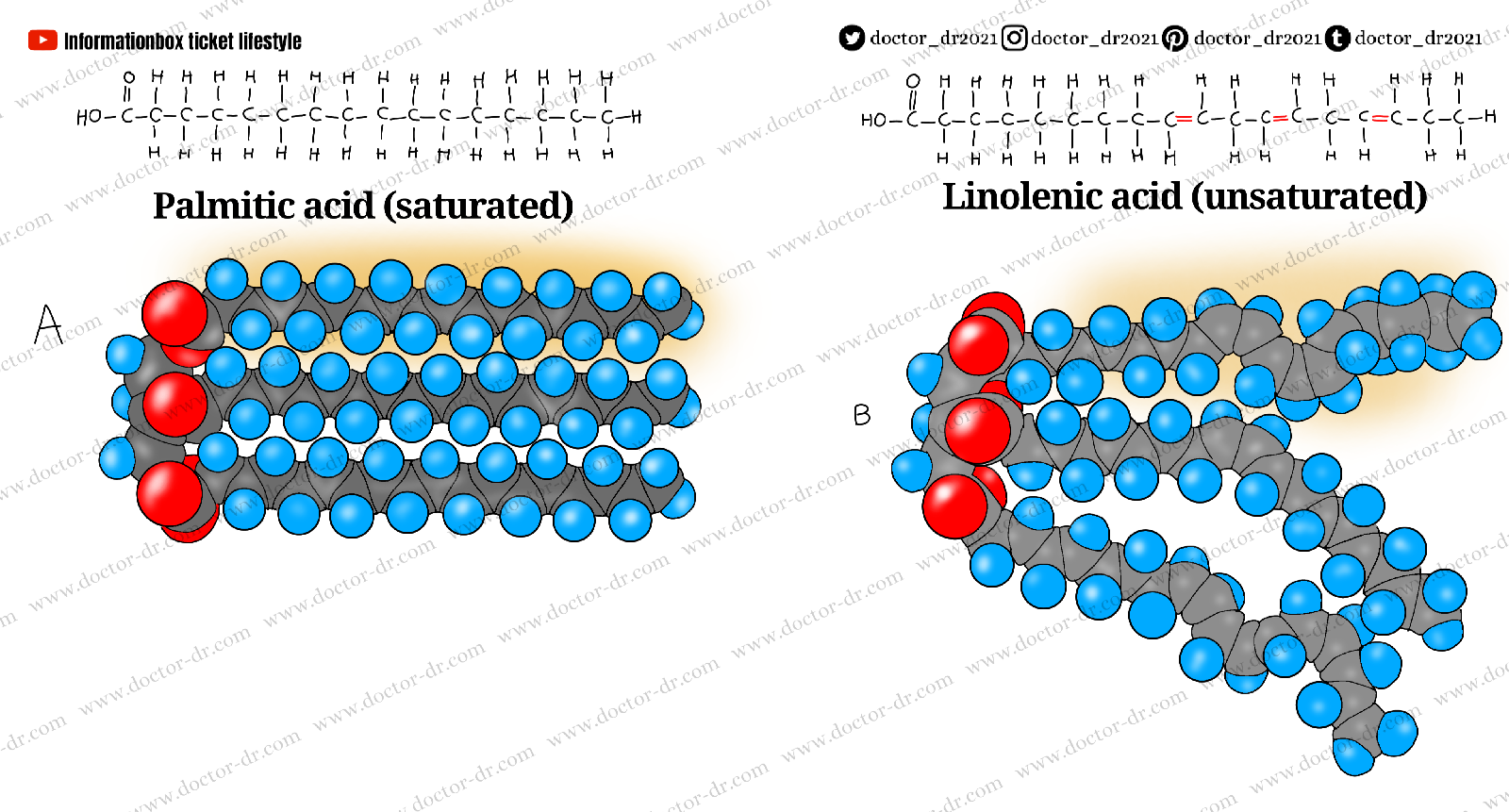
Fatty acid classifications. Fatty acids differ in the amount of hydrogen atoms connected to, or "saturate," the accessible bonds surrounding each carbon in the chain, as well as the length of their carbon chains (number of carbon atoms). A structural formula and three-dimensional model for a saturated (palmitic) and unsaturated (linolenic) fatty acid.
A saturated fatty acid is one in which all of the accessible hydrogen atoms in its hydrocarbon chain have been filled, or saturated. There are no double bonds in the chain. Because not all of the chain carbon atoms are saturated with hydrogen atoms, an unsaturated fatty acid has one or more double bonds in its hydrocarbon chain. The degree of saturation is the most critical element in defining fatty acids' physical and chemical properties. At normal temperature, animal fats like tallow and lard are solids, whereas vegetable oils are liquids. The difference is in the amount of unsaturation in animal fats vs vegetable oils. The presence of double bonds in a fatty acid molecule causes the chain to "kink" or bend.
As the amount of unsaturated double bonds in a fat rises, it becomes more oily and liquid. The unsaturated molecules' "kinks" and "bends" prevent them from fitting together tightly. Saturated fatty acids, on the other hand, lack kinks, allowing the molecules to fit firmly together and form a solid mass at higher temperatures.
Triglyceride synthesis. The creation of a triglyceride is seen in Figure 2-17. Its name, glycerol tristearate, implies that it is made up of three stearic acid molecules linked to a glycerol molecule. The three stearic acid "building blocks" connect to the three hydroxyl groups (OH) of the glycerol molecule through their carboxyl groups (COOH), producing the triglyceride and three molecules of water. It's a dehydration synthesis reaction, which you're probably acquainted with. Keep in mind that while certain fats, such as glycerol tristearate, have three molecules of the same fatty acid linked to glycerol, others may have two or three distinct fatty acids.
Phospholipids
Phospholipids are fat molecules that resemble triglycerides. However, one of the three fatty acids linked to glycerol in a triglyceride is replaced by another type of chemical structure including phosphorus and nitrogen in a phospholipid. Figure 2-18 depicts the structure of a phospholipid mula. Glycerol is included in the phospholipid molecule. Two fatty acids are attached to the glycerol at one end of the molecule. The phosphate group, which is linked to a nitrogen-containing molecule, is attached to glycerol but extends in the other direction.
In a phospholipid, the end of the molecule that contains the phosphorus group is water soluble, whereas the end formed by the two fatty acids is fat soluble. Phospholipid molecules have the unusual feature of being able to connect or bridge two separate chemical environments: a water environment on one side and a lipid environment on the other. As a result, phospholipids are an essential component of cell membranes, and they will be addressed in further depth in Chapter 3.
FIGURE 2-18 Phospholipid molecule. A, Chemical formula of a phospholipid molecule. B, Molecular model showing water and fat-soluble regions. C, The way phospholipids are often depicted. D, Orientation of phospholid molecules in an oil-water interface. E, Orientation of phospholid molecules when surrounded by water.
Steroids
Steroids are a broad category of chemicals whose molecules include the steroid nucleus as their main component (Figure 2-19). Steroids are extensively dispersed throughout the body and play a variety of structural and functional roles. Cholesterol is a steroid that is present in the plasma membrane of every cell in the body (see Chapter 3). Its presence aids in the stabilisation of this critical cellular structure and is necessary for many of the processes that cells must carry out in order to live. Cholesterol and other steroid compounds are also needed to make hormones including oestrogen, testosterone, and cortisol.
FIGURE 2-19 The steroid nucleus. The steroid nucleus-highlighted in yellow-found in cholesterol (A) forms the basis for many other important compounds such as cortisol (B), estradiol (an estrogen) (C), and testosterone (D).
Prostaglandins
Prostaglandins are lipids made up of a 20-carbon unsaturated fatty acid with a 5-carbon ring. They're also known as "tissue hormones." In the body, there are many distinct types of prostaglandins. We currently divide 16 prostaglandin din types (PGs) into nine major groups, referred to as PGA through PGI. Based on chemical structure and function, each main grouping of prostaglandins can be further split.
Prostaglandins were initially discovered in prostate tissue and given the name "prostaglandin." However, further research has revealed that cell membranes in nearly every bodily tissue generate these physiologically potent chemical compounds. In response to a specific stimulus, they are produced and subsequently released from cell membranes. They have a relatively local impact once released, and then they are inactivated.
The effects of prostaglandins in the body are many and varied. They play a crucial role in regulating the effects of several hormones, influence blood pressure and secretion of digestive juices, enhance the body immune system and inflammatory response, and play an important role in blood clotting and respiration, to name a few. The use of prostaglandins and prostaglandin inhibitors as drugs is an exciting and rapidly growing area in clinical medicine. Treatment of specific disease states, symptoms, or medical conditions using prostaglandins er drugs that inhibit prostaglandin action ranges from their use to relieve menstrual cramps to treatment of asthma, high blood pressure, and ulcers.
QUICK CHECK
1. What are the building blocks of a triglyceride or fat?
2. Give an example of a dehydration synthesis reaction.
3. What is a phospholipid, and why is it an important molecule?
4. Identify an important steroid.