Table of Contents
- Structure of Herpes simplex virus 1 (HSV-1)
- Genome of Herpes simplex virus 1 (HSV-1)
- Epidemiology of Herpes simplex virus 1 (HSV-1)
- Transmission of Herpes simplex virus 1 (HSV-1)
- Replication of Herpes simplex virus 1 (HSV-1)
- Pathogenesis of Herpes simplex virus 1 (HSV-1)
- Clinical manifestations of Herpes simplex virus 1 (HSV-1)
- Laboratory Diagnosis of Herpes simplex virus 1 (HSV-1)
- Treatment of Herpes simplex virus 1 (HSV-1)
- Prevention and Control of Herpes simplex virus 1 (HSV-1)
The Herpes simplex virus 1 structure (HSV-1)
- Herpesviruses have an icosahedral symmetry and are big (150–200 nm in size).
- The double-stranded DNA genome contains 125–240 kbp nucleotides, and 162 hollow hexagonal and pentagonal capsomeres make up the nucleocapsid, which together with the icosahedral protein capsid has an average width of 100 nm.
- The membrane that surrounds the nucleocapsid is made of lipoproteins.
- The nuclear membrane of the infected recipient cell serves as the source of the lipid component.
- Virus entrance is mediated by spikes of viral glycoproteins, which are 8 nm long and project from the trilaminar lipid host-derived envelope.
- HSV has at least 11 glycoproteins that are used for various functions, including (a) virus attachment proteins (gB, gC, gD, and gH), (b) fusion proteins (gB), (c) structural proteins, (d) immune escape proteins (gE, and gI), and (e) other fractions.
- The tegument, an amorphous proteinaceous layer outside the capsid of mature viral particles, is encircled by a lipid envelope made of host cell membranes.
- The tegument is made up of enzymes like VP16, which subverts cellular proteins, enzymes involved in viral nucleic acid reproduction, and VHS (Virion Host Shut off), which stops the host cell's cytoplasmic protein synthesis.
Genome of Herpes simplex virus 1 (HSV-1)
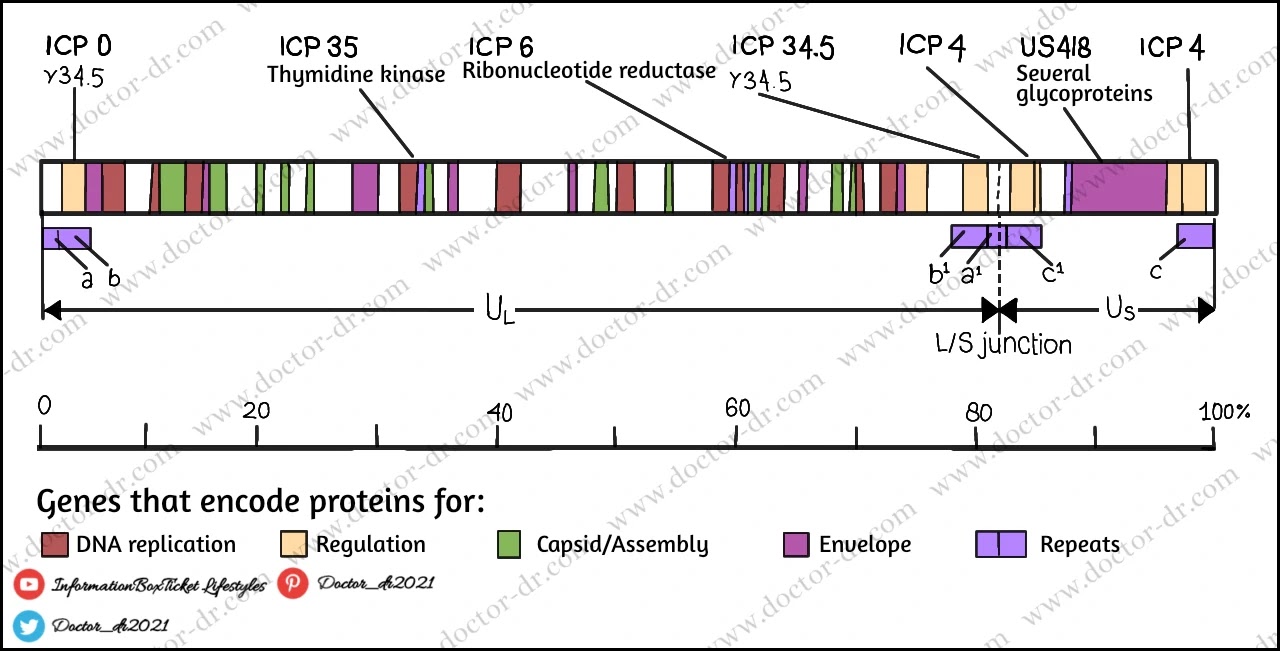
The herpes simplex virus genetic map. A single, linear molecule of double-stranded DNA, measuring about 152,000 bp in length, makes up the HSV-1 genome. It is split into two distinct sections known as long (U L) and brief (U S ). At the ends of the genome and in between the L and S segments, there are brief sections of repeated sequence (a/b/c and a'/b/c'). There are four distinct genome isomers produced as a result of the rapid inversion of the L and S regions during DNA replication. In the majority of groups of wild-type HSV-1, all four are equally prevalent.
- The virus's straight, double-stranded DNA genome has a molecular weight between 125 and 240 kbp.
- There are between 60 and 120 genes in the large herpesvirus genome, which encodes at least 100 distinct proteins.
- At least 10 of these polypeptides make up the viral envelope, and more than 35 are involved in the formation of the virus particle.
- Herpesviruses contain a variety of virus-specific enzymes involved in the control of protein synthesis, gene expression, nucleic acid metabolism, and DNA synthesis (DNA polymerase, helicase-primase, thymidine kinase, transcription factors, protein kinases).
- Herpesvirus DNAs have a striking sequence arrangement with internal and terminal repetitive sequences.
- It is broken down into 6 types—A, B, C, D, E, and F—based on how the order is arranged.
- The Herpes Simplex virus contains a type E DNA.
- Two components make up the termini of class E.
- The unique sequences are divided into long (Ul) and short (Us) domains by the terminal sequences (ab and ca), which are introduced inverted.
Epidemiology of Herpes simplex virus 1 (HSV-1)
- Herpes simplex viruses are equally distributed in both sexes and throughout the globe, with no seasonal variation.
- With 65% of people in the US having HSV-1 antibodies, HSV-1 infection is more prevalent than HSV-2 infection.
- Similar distribution exists in Europe, where at least 50% of the population is HSV-1 seropositive.
- HSV-1 is almost always contracted in the developing world during early infancy through close contact with family members.
Transmission of Herpes simplex virus 1 (HSV-1)
- Saliva is the mouth route of HSV-1 transmission.
- It is typically spread through oral contact, such as kissing or sharing toothbrushes or other objects contaminated with saliva.
- A minor skin abrasion can allow the HSV virus to infiltrate the body and cause an infection after mouth-to-skin contact.
- Eye infections can also result from autoinoculation.
Replication of Herpes simplex virus 1 (HSV-1)
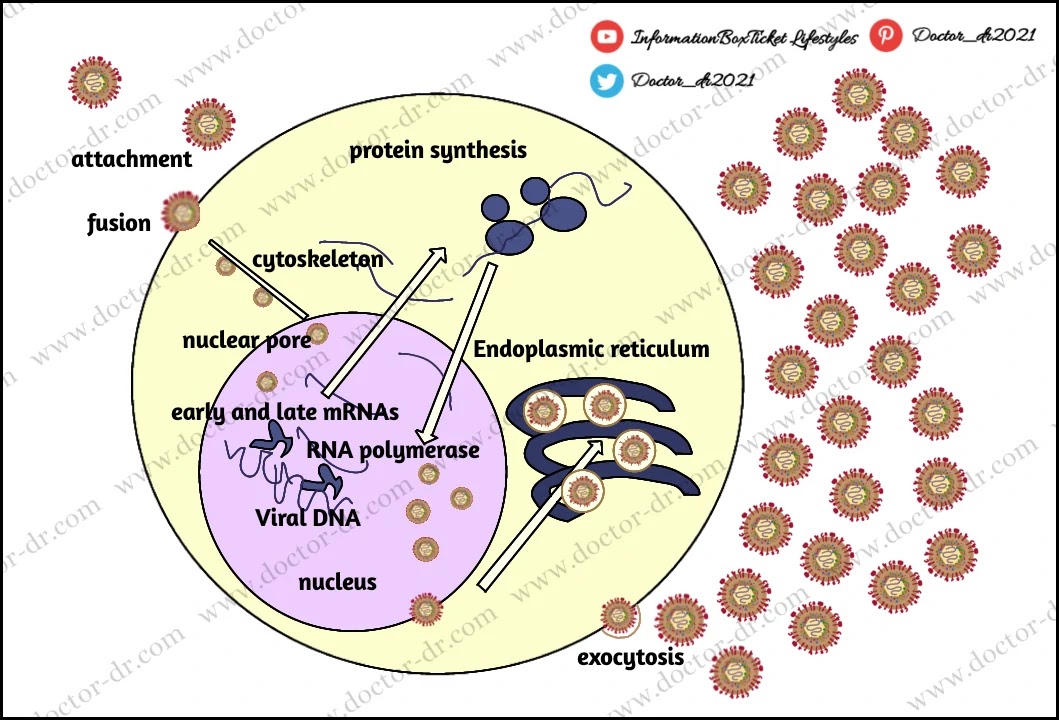
- A number of co-receptors, including HveA (Herpes virus entry mediator A, also known as HVEM, Herpes Virus Entry Mediators), must be activated by gD in order for HSV1 to enter cells. This is followed by the fusion of the viral envelope with the cell plasma membrane and the delivery of the viral capsid into the cell cytoplasm.
- The virus proteins VHS and VP16 are also released in the cytoplasm along with the capsid proteins.
- After being propelled to the nucleopore for entry into the nucleus, the inbound viral capsids are then destroyed, releasing only DNA into the nucleus.
- For viral transcription and replication in the nucleoplasm, the viral DNA is left uncoated.
- The two major stages of transcription are early, which occurs before genome replication, and late, which occurs after replicated genomes are found in virus replication compartments created in the nucleus of the infected cell.
- Alpha, Beta, and Gamma types of mRNAs are produced, and their regulation occurs in a coordinated cascade.
- The primary transcriptional regulatory proteins are found in the Alpha or IE (Immediate-Early) genes, and the transcription of the Beta and Gamma gene classes depends on the synthesis of these proteins.
- A DNA polymerase, a single-strand DNA-binding protein, a primosome or helicase-primase, an origin-binding protein, and a group of enzymes involved in DNA repair and deoxynucleotide metabolism are all included in the beta proteins, which are necessary for the virus genome to replicate.
- The temporal programme of viral gene expression ends with the appearance of the Gamma or late proteins, which make up the structural proteins of the virus. Viral DNA synthesis starts soon after the appearance of the Beta proteins.
- Soon after infecting susceptible host cells, the linear 153 Kb pair genome circularises and enters a rolling circle phase of DNA replication, producing branched concatameric DNA. This DNA is then cleaved to release linear ds DNA.
- In the nucleus, viral transcription and DNA duplication take place. The particle then assembles and leaves epithelial cells in the skin, where it causes a primary infection.
- Virion budding through the nuclear membrane is how the virion obtains its envelope.
Pathogenesis of Herpes simplex virus 1 (HSV-1)
- By kissing or sharing saliva, HSV-1 can be transmitted.
- The virus is typically picked up during sexual behaviour or during childhood, either through oral-oral or oral-genital contact.
- Epithelial cells are infected by HSV-1, and infection starts when viral particles bind to the cells.
- Through glycoproteins that extend from the viral membrane, virions communicate with particular cell-surface receptors.
- The vesicle, a ballooning degeneration of intraepithelial cells containing infectious fluid, is the usual lesion caused by HSV.
- Multinucleate cells (Tzanck cells) make up the base of the vesicle, and the nuclei of contaminated cells have eosinophilic inclusion bodies.
- An ulcer develops after the vesicle's covering disintegrates.
- On mucous membranes and non-keratinizing epithelia, this occurs quickly; on the epidermis, the ulcer forms a scab, crusts over, and then heals.
- Natural killer (NK) cells are important in the early stages of protection because they identify and eliminate HSV-infected cells.
- The three distinct biochemical characteristics of HSV are neurovirulence, latency, and reactivation.
- Following local site of incoluation infection, the virus invades nearby nerve endings before travelling via retrograde axonal flow to the dorsal root ganglia, where it continues to multiply before entering latency.
- Most primary HSV infections are asymptomatic and minor in nature.
- Latency, which refers to a non-replicating condition, occurs in the trigeminal ganglia.
- Except for a small RNA called micro-RNA (encoded by a latency-associated viral gene), which maintains the latent infection and prevents cell death, HSV does not replicate in the latent state.
- Reactivation mechanisms continue to be poorly known.
- It has been hypothesised that HSV DNA travels along the nerve axon returning to the nerve ending, where epithelial cell infection may take place.
- Not all reactivation will cause a visible lesion; some cases of reactivation may involve asymptomatic viral shedding that can only be identified using culture or DNA detection techniques.
- There is still much to learn about the variables affecting the growth of recrudescent tumours.
- At the period of recurrences, CD8+ T suppressor lymphocyte activity frequently increases.
- HSV transmission may be facilitated by some mediators (such as prostaglandins), a transient decline in immune effector cell function, especially delayed hypersensitivity.
- A local rise in prostaglandin levels is undoubtedly associated with known recurrence triggers, and the suppression of cell-mediated immunity makes herpes recurrence more likely.
- It happens normally and can be brought on by a number of stimuli, including fever, trauma, worry, ultraviolet light (sunlight), and anxiety.
- It has been repeatedly shown in patients who are having neurological interference with their trigeminal ganglion, a frequent site of herpes latency, that the time between the stimulus and the development of a clinically obvious lesion is 2–5 days.
Clinical manifestations of Herpes simplex virus 1 (HSV-1)
A. Oropharyneal disease
- Symptomless primary HSV-1 infections are the norm.
- Small children (1–5 years old) are most commonly affected by this symptomatic illness, which affects the buccal and gingival mucosa of the mouth.
- On the tongue and in the front of the mouth, vesicular sores ulcerate quickly (stomatitis).
- The most noticeable and frequent lesion is gingivitis (swollen, sensitive gums).
- Pharyngitis and tonsillitis are prevalent among adults who have primary infections.
- Additionally, cervical lymphadenopathy and herpetic dermatitis (vesicles on the lips and tissue around the mouth) can happen.
- A collection of vesicles, typically found at the lip's edge, is what distinguishes recurrent illness.
- The pustular and crusting phases of a lesion are progressed through, and in 8–10 days, the healing process without scarring is typically complete.
- The lesions may come back in the same place frequently and at different times.
- Individuals experience recurrences at varying rates.
B. Keratoconjunctivitis
- HSV infection of the eye can manifest as periorbital vesicles on the eyelids, keratoconjunctivitis accompanied with corneal ulceration, or conjunctivitis and conjunctivitis.
- The corneal stroma may gradually become involved with recurrent keratitis, leading to irreversible opacification and blindness.
C. Skin infections
- HSV-1-infected abrasions may develop localised sores in the form of the virus (traumatic herpes).
- These lesions, known as herpetic whitlow, are typically found on the fingers of dentists and medical professionals, but they can also appear elsewhere, including on the bodies of fighters as a result of close skin-to-skin contact, where they are known as herpes gladiatorum.
D. Eczema herpeticum
- When skin conditions like eczema or burns, which allow for widespread local viral replication and spread known as eczema herpeticum or Kaposi's varicelliform eruption, are present, cutaneous infections are frequently severe and life-threatening.
- Dehydration and protein loss are caused by extensive ulceration, and viraemia can cause disseminated illness with serious, even fatal, effects.
E. Meningitis/ encephalitis
- Infections with HSV-1 are thought to be the most typical source of fatal, sporadic encephalitis in the US.
- The illness has a high mortality rate, and survivors frequently still have neurologic abnormalities.
- One option is direct infection from the nasal mucosa along the olfactory tract, but central spread from the trigeminal ganglia is the most probable one.
F. Genital herpes
- Although HSV-2 is primarily responsible for genital herpes, HSV-1 can also result in some clinical cases.
- The typical duration of an illness from a primary genital herpes virus is three weeks.
- Vesiculo ulcerative sores of the male penis or the female cervix, vulva, vagina, and perineum are the hallmarks of genital herpes.
- Malaise, dysuria, and discomfort accompanied by fever are among the signs and symptoms.
G. Neonatal herpes
- A newborn's HSV infection can develop in utero, during delivery, or after birth.
- In every instance, the mother is the most frequent source of infection.
- Postnatal contact to HSV-1 or HSV-2 can result in the postnatal development of neonatal herpes.
- Contact with herpetic lesions in the birth canal is the most typical way for HSV to infect a neonate during birth.
- For pregnant women with genital herpes lesions, caesarean delivery has been used to prevent transmission.
Laboratory Diagnosis of Herpes simplex virus 1 (HSV-1)
Specimens
- oral sample, skin swab, corneal scrapings, skin scrapings, vesicle swab, vesicle fluid, blood, tissue, and CSF
Culture
- For the separation of viruses, tissue cultures are injected.
- HSV is simple to grow, and cytopathic consequences typically appear in just two to three days.
- In cell cultures of fibroblasts and epithelial kinds, the virus can quickly proliferate and cause the cell to balloon and become grounded.
- Then, using a neutralisation test or immunofluorescence staining with a particular antiserum, the agent is determined.
Cytopathology
- By using Giemsa or Wright's stain, also known as a Tzanck smear preparation, to colour scrapings taken from the base of vesicles, cytopathologists can identify multinucleated giant cells.
- HSV infection can be identified by the presence of typical giant cells or bovine dry type A intranuclear inclusion bodies in the stained smear.
Antigen detection
- Direct fluorescent antigen detection and direct enzyme assays can be used to find the antigen in vesicle fluid, tissue smears, and biopsy samples.
Antibody detection
- After the infection, antibodies start to develop in 4–7 days and reach their climax in 2–4 weeks.
- ELISA, IFT, and Complement fixation tests can be used to check for the existence of IgM or a rising IgG titre in order to identify primary infections.
- HSV-1 and HSV-2 can be distinguished by serologic tests based on the type-specific antigens, glycoprotein G.
Molecular Diagnosis
- The most accurate method for identifying HSV DNA and a method that can be used to distinguish between HSV-1 and HSV-2 is the polymerase chain reaction (PCR).
Treatment of Herpes simplex virus 1 (HSV-1)
- Acyclovir has a higher treatment ratio and has been shown to be effective.
- The HSV thymidine kinase monophosphorylates the nucleoside analogue acyclovir, which cellular enzymes then change into the triphosphate form.
- The HSV polymerase effectively incorporates the acyclovir triphosphate into the virus DNA, where it stops chain elongation.
- In addition to acyclovir, valacyclovir and vidarabine are also used to stop the production of DNA.
Prevention and Control of Herpes simplex virus 1 (HSV-1)
- The HSV virus does not have a vaccine.
- Taking basic personal hygiene precautions and reducing crowding can reduce the spread of herpes simplex.
- instruction on the phases of infection.
- utilising contraceptives.