Table of Contents
1. Galacturonans1. Pectinolytic bacteria
1. Polygalacturonase
2. Pectinesterase
1. Moisture content
2. Aeration
3. Added glucose
1. Deesterification
1. Mechanism of de-esterification by pectin esterases and pectin methyl esterases
Example
2. Mechanism of hydrolytic cleavage in polygalacturonases and pectin lyases
What is pectin?
- Pectin, a complex heteropolysaccharide used as a cement in plant cell walls, is made up of linear chains of -D-galacturonic acid or other comparable sugar derivatives.
- Pectin frequently continues to be linked to other polysaccharides found in cell walls, such as cellulose, hemicelluloses, and lignin.
- The main cell wall and middle lamella of plant cells have the largest concentrations of pectin, whereas the concentration decreases towards the plasma membrane.
- Pectin is in charge of giving the cell wall stiffness and structure. It also aids in intercellular adhesion and the mechanical resistance of the cell.
- Although some types of non-soluble or bound pectin can also be discovered, the majority of natural pectin is water-soluble or free.
- The length of the polymer and the presence of a methoxy group in the structure determine how soluble pectin is.
- Pectin has a wide range of commercial uses since it may exist as a thick, gel-like structure.
- Due to its high content of dietary fibres that may be fermented, pectin is one of the few biopolymers that are researched.
- Due to the intricacy and variety of their structural makeup, pectin has several uses.
- The word "pectin," which meaning "curdled or congealed," is derived from the Greek word "pektikos."
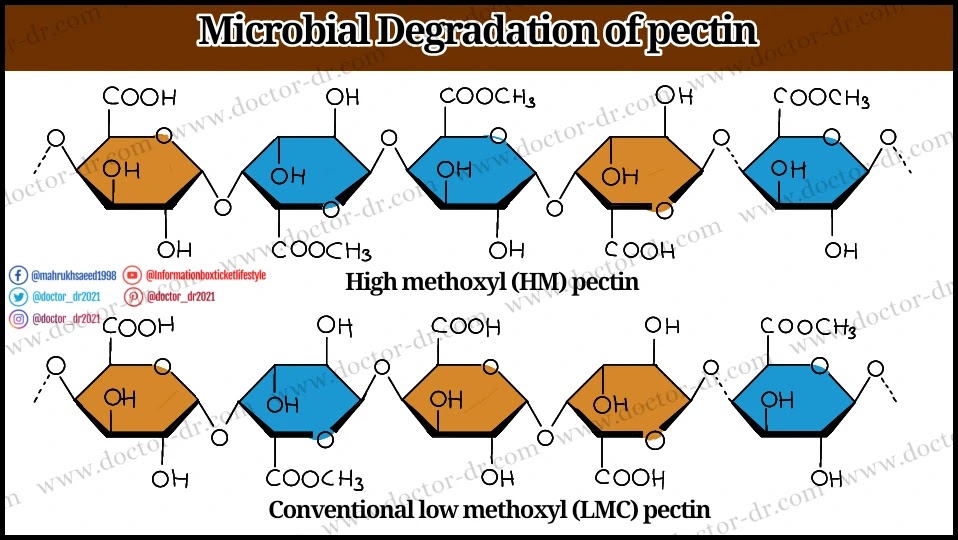
Structure of pectin
- A class of galacturonic acid-rich plant cell wall polysaccharides known as pectins is covalently linked.
- Galacturonic acid is present in approximately 70% of all pectin, and it is connected to all pectin polysaccharides at the O-1 and O-4 locations.
- Galacturonans and rhamnogalacturonans are two groups into which the structural components of pectin are divided.
- Galactorunans have a backbone made of D-galacturonic acid residues that are -(1,4) connected. There are two types of branching: branched and unbranched.
- However, rhamnogalacturonans have diglycosyl repeating units of -L-rhamnose-(1,4)-D-galacturonic acid in their backbone.
- The polymeric side chains, which also comprise arabinose and galactose residues at other places, ramify the rhamnose residues at the O-4 and O-3 sites.
- Four distinct forms of polymeric side chains—galactans, type I arabinogalactans, and type II arabinogalactans—can be found in pectin.
- Pectin has a very complex chemical structure since it may include up to 18 distinct monosaccharides connected by 20 different links.
- Pectin's general structure is described in terms of its hairy and smooth sections. While the hairy area has simple or complicated side chains, the smooth sections have homo- or heteropolymer linear chains.
- Besides, several other monosaccharides might remain bonded by modified O-ether or O-ester linkages.
- There are two types of galacturonans that may be discovered in pectin: homogalacturonans and heterogalacturonans.
- Homogalacturonans, which are unbranched chains of -(1,4)-linked G-galacturonic acid residues that may be methyl or acetyl-esterified, make up the smooth portion of pectin.
- About 60% of the pectin discovered in various living things is hogalacturonans.
- Axylogalacturonan is produced in the case of heterogalacturonanas when the homopolysaccharide chain is more or less significantly replaced at O-2 and O-3 by monomers or dimers of xylose.
- Rhamnogalacturonans are produced when the polymer is replaced with complicated side chains like rhamnose.
2. Rhamnogalacturonans
- Some of the pectins might have L-rhamnose and D-galacturonic acid residues arranged in a long chain called rhamnogalacturonan.
- In rare instances, other side chains including L-arabinosyl and D-galactosyl might potentially take the place of the rhamnose residues.
- There might be a few glucuronic acid and 4-O-methyl glucuronic acid residues.
- In nature, rhamnogalacturonans make up 20–35% of the total pectin content, while the percentage can reach 75% in the soybean plant.
What are Pectinases?
- Pectinases are a collection of enzymes that break down pectin from diverse sources. There are at least seven distinct pectinases in total.
- Pectinases are varied because various live organisms contain a variety of pectins.
- The most prevalent and crucial pectinases for industry are grouped into various classes based on variations in their substrate, structure, and reaction mechanism.
- Pectinesterases, polygalacturonases, pectin lyases, and pectin depolymerases are a few of the common pectinolytic enzymes.
- Due to their role in the extraction and purification of juice as well as the maceration of plant tissues, pectinases have significant industrial uses.
- One of the key enzymes that participate in the global carbon cycle and help recycle natural waste is pectinases.
- Since pectin compounds are broken down into saturated and unsaturated galacturonans, which may subsequently be catabolized to generate either pyruvate or 3-phosphoglyceraldehyde, pectinases are even referred to as carbon recycling enzymes in nature.
- Pectinases are also employed in degumming fibre crops, as an enzyme complex for making animal feed, for filtering plant viruses, and for oil extraction in addition to these purposes.
- Some pectinases function as virulence factors because they aid in the breakdown of the pectin contained in plant cells.
- Depending on their source, pectinases may differ in structure and action. For example, a pectinase from a fungus may differ from a pectinase from a bacterium.
- Fungal pectinases are acidic in nature, but bacterial pectinases are often alkaline.
Microorganisms involved in pectin degradation (pectinolytic microorganisms)
It is known that many kinds of microbes create various pectinolytic enzyme sets that either benefit in nutrient absorption or aid in the development of microbial illnesses.1. Pectinolytic bacteria
- In recent years, bacteria have emerged as a significant source of pectinolytic enzymes, producing a variety of sets of enzymes that aid in the general breakdown of pectin substrates.
- Bacillus, Pseudomonas, and Staphylococcus are a few examples of frequent pectinolytic bacteria.
- Aerobes can detect the majority of bacterial pectinolytic activity in aerobic circumstances, but some activity may be detected under anaerobic conditions.
- Some bacteria, including Pseudomonas aeruginosa, Bacillus psychrosaccharolyticus, Bacillus badius, and Bacillus asahin, even use the pectinolytic activity in their pathogenesis of various illnesses.
- Geobacillus sp., Anoxybacillus sp., and Bacteroides are common thermophilic bacteria that also have pectinase activity, helping the biosphere recycle carbon molecules.
2. Pectinolytic fungi
- The majority of microorganisms that participate in the natural recycling process of polysaccharides are fungi.
- These fungus may have a variety of environments and behaviours.
- The Ascomycetes and Deuteromycetes species are the most prevalent fungus engaged in pectin breakdown.
- One of the most researched basidiomycetes (white rot) fungus, Phanerochaete chrsosporium, breaks down the majority of complex polysaccharides, including cellulose, pectin, and chitin.
- Magnaporthe oryzae, Giberella zeae, Botrytis fuckeliana, Sclerotinia sclerotiorum, Aspergillus nidulans, Trichoderma virens, Podospora anserine, Rhizopus oryzae, and Aspergillus clavatus are further fungi involved in pectin breakdown.
- The sort of enzymes generated and how they work may vary depending on the fungus species.
Enzymes involved in the degradation of pectin
Pectinases are divided into many classes based on the source of the enzymes, their substrates, and the reaction process;
1. Polygalacturonase
- A class of enzymes known as polygalacturonase hydrolyze O-glycosyl linkages in homogalacturonan to produce monomeric units.
- These enzymes work on the galacturonic residues' 1,4-D-galactosyluronic connections.
- The majority of polygalacturonases are endo-enzymes that randomly act on links to depolymerize the chain or shorten the polymer.
- Homogalacturonan is the endo-polygalacturonase's natural substrate; however, other substances, such as oligogalacturonides, may also function as a substrate, depending on the substrate.
- There is also a kind of exo-polygalacturonase that converts polygalacturonates into di- and mono-galacturonates.
- Measuring the amount of reducing sugars produced as a result of hydrolysis or using the viscous reduction method both serve to gauge the enzyme's activity.
2. Pectinesterase
- A class of enzymes known as pectinesterases is responsible for hydrolyzing the methylated carboxylic ester found in pectin to produce pectic acid and methanol.
- Pectin is the natural substrate of pectinesterase, but it may also take up other substances including methyl pectate and methylation oligogalacturonides.
- (NH4)2SO4, Mg2+, and NaCl all increase or promote pectinesterase activity. The presence of Cu2+ and Hg2+ inhibits it.
- The majority of the pectinesterase that has been thoroughly examined comes from plants, although more recently, bacterial and fungal sources of pectinesterase have also been identified.
- The majority of pectinases have a preference for esterified pectic compounds and may not be active towards pectates.
3. Pectin lyases
- Unsaturated oligomethylgalacturonates are produced in a 4:5 ratio as a result of the random degradation of pectin compounds by pectin lyases.
- Through a transelimination process, these enzymes selectively cleave glycosidic bonds on polygalacturonic acid.
- Chelating chemicals like EDTA are effective in inhibiting pectin lyases because they have a strict demand for Ca2+ ions.
- The cleavage of the substrate from the non-reducing end of the polymer is catalysed by exo-pectin lyases.
Factors affecting pectin degradation
Different factors, including some of the following, can impact pectin breakdown in nature and on artificial growth medium.
1. Moisture content
- Based on research on the degradation of pectin, it has been found that the presence of free water and total saturation accelerates the rate of chitin breakdown.
- The rate of pectin breakdown is very slightly impacted by changes in water concentration or moisture content.
- However, the rate is compromised if the water level rises to the point when logging compromises aeration.
2. Aeration
- The majority of pectinolytic bacteria are facultative or aerobic aerobics. As a result, the higher O2 concentration accelerates the rate of pectin utilisation or breakdown.
- However, because a low concentration of CO2 permits facultative aerobes and anaerobes to continue their activity, some degree of degradation can still occur.
- 100% oxygen environments can occasionally be harmful, especially when easily accessible energy sources are present.
3. Added glucose
- As the organisms use the readily accessible source of energy rather than pectin as their supply of nutrients, the addition of glucose to the medium or soil slows the rate of pectin decomposition.
- Glucose is a readily available and simple to metabolise energy source. Pectin degradation is subsequently delayed or reduced as a result of this.
- However, because pectin is significantly less complex than other carbohydrate sources like lignin, pectin breakdown is accelerated in the absence of glucose or other comparable sources.
4. Organic matter
- The rate of pectin breakdown is further supported by the presence of plant fibres rich in pectin.
- Proteins and enzymes are formed by microbes using organic matter that is rich in nutrients and minerals.
- The concentration of substrate rises as organic matter increases. As the organisms use sources like glucose and cellulose, the increasing substrate content may at first cause the breakdown rate to drop.
- Pectin becomes the next source of nourishment when these other sources deteriorate, which accelerates pectin's deterioration.
Process (Simple Steps) of pectin degradation
The microbial hydrolysis or degradation of pectin in nature occurs in the form of the following steps;
1. Deesterification
- Pectin esterases or pectin methyl esterases are the first enzymes to interact with pectin-containing compounds.
- Pectic acid and methanol are produced as a result of these enzymes' catalyzation of the desesrerification of the methoxy group in pectin.
- Because they require non-esterified substrates, esterase enzymes function before polygalacturonates and pectate lyases.
- A methyl ester group of galacturonate units is preferred by pectin esterases over a galacturonate unit that has not been esterified.
- Pectin's acetyl ester is hydrolyzed by esterases like pectin acetyl esterases, producing pectic acid and acetate.
2. Hydrolytic cleavage
- The hydrolytic breakage of the -1,4-glycosidic links found in the pectin substrates' backbone is the most significant step in pectin breakdown.
- To do this, various bacteria develop various enzymes, each of which acts on a distinct subset of pectin substrate.
- Highly esterified pectin's -1,4-glycosidic linkages are affected by polymethylgalacturonases and polygalacturonases, resulting in 6-methyl-D-galacturonate and D-galacturonate, respectively.
- Both of these enzymes are capable of cleaving the pectin backbone randomly or via the reducing ends as endo- or exoenzymes.
- Pectate lyases are a different class of hydrolytic enzymes that operate on the glycosidic bonds in polygalacturonic acid to produce unsaturated products through a transelimination process.
Mechanisms of microbial degradation of pectin
- The type of enzyme used in the process affects how pectin is broken down by microbes.
- The following are a few examples of pectin breakdown enzymes' mechanisms of action:
1. Mechanism of de-esterification by pectin esterases and pectin methyl esterases
One of the three processes is used by pectin esterases to interact with the pectin substrates;
- the single-chain method, in which the enzyme interacts with the polymeric chain's whole substrate side.
- the multiple-chain mechanism that starts with the catalysis of just one reaction and ends with the substrate being dissociated.
- before the enzyme-substrate complex separates, the enzyme catalyses a number of processes using a multiple-attack mechanism.
- Bacterial polyesterases use both a single-chain and a multiple-attack method to create products having contiguous regions of galacturonic acids.
- However, fungal esterases attack at random via a multiple-chain strategy.
- De-esterification during the random assault results in the release of protons, which encourages the activity of endopolygalacturonases.
Example
In Bacteroides, the multi-attack method used to de-esterify galacturonan macromolecules is followed by the breakdown of the oligomers to release the final products.
2. Mechanism of hydrolytic cleavage in polygalacturonases and pectin lyases
- The placement of the active site amino acids on the vulnerable glycosidic bonds is the first step in the hydrolytic cleavage of 1,4-glycosidic bonds in pectin.
- Through a series of hydrogen bonds, the motifs on the active sites communicate with the substrate on either side of the specified bond.
- The susceptible glycosidic bond is sufficiently strained and distorted by the hydrogen bonds.
- Proton transfer between the amino acids in the active site and the glycosidic link occurs after the distortion.
- This results in the release of the first end product and the breaking of glycosidic bonds. Subsequently, a covalent link is formed between the substrate and the nucleophile at the catalytic site.
- A water molecule is then positioned on the enzyme's active site in preparation for a nucleophilic assault on the substrate. The second end product is produced as a result of the nucleophilic attack, and the enzyme's active site is also restored.
Example
About 18 polygalacturonases and one -galactosidase are involved in the hydrolytic cleavage process in Rhizopus oryzae. These enzymes break down the 1,4-glycosidic bonds via the aforementioned endo- and exoenzyme method.




%20-%20Symptoms,%20Treatment,%20and%20Management.webp)
