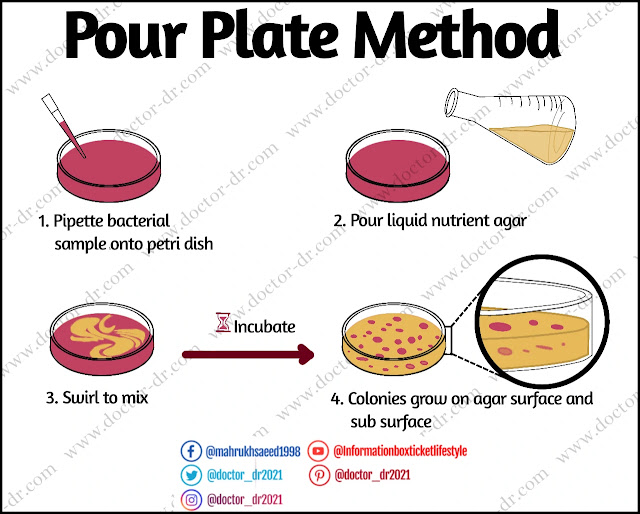
Robert Koch developed the pour Plate Method method, which is still commonly used today. This approach works well with facultative, microaerophilic, and anaerobic bacteria. It is simple, less resource-intensive, simple, and inexpensive; nevertheless, the sample must be in liquid or suspension.
Table of Contents
- What is Pour Plate Method?
- Objectives of Pour Plate Technique
- Principle of Pour Plate Method
- Requirements for Pour Plate Method
- Procedure of Pour Plate Method
- Result Interpretation of Pour Plate Method
- Precautions during Pour Plate Technique
- Applications of Pour Plate Technique
- Advantages of Pour Plate Technique
- Limitations of Pour Plate Technique
What is Pour Plate Method?
The pour plate method is a microbiological laboratory technique for identifying and counting live bacteria in a liquid sample that is introduced with or before molten agar media before solidification.
In general, this approach is used to count viable microorganisms in a particular sample by counting the total number of colony-forming units (CFUs) within and/or on the surface of the solid medium. It is mostly used to count bacteria, however Actinobacteria, molds, and yeasts can also be separated and counted.
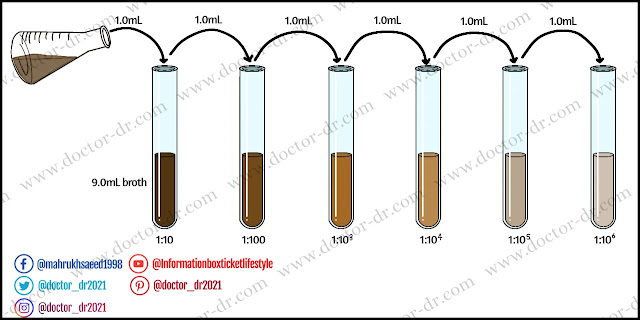
Prior to using the pour plate technique, the sample must be serially diluted to achieve a microbial load of 20 - 300 CFU/mL. (suitable colony counting range is 20 – 200, some consider it to 30 – 300, and in average it is taken as 25 – 250). If the material is a liquid, it can be diluted serially with sterile distilled water or sterile broth. If the sample is solid or semisolid, it must first be emulsified before being serially diluted to lower the microbial load to the allowable range.
The sample is either placed to the Petri plate and then poured with the molten agar medium, or the sample is mixed with the molten agar media before pouring. After putting the sample into the Petri plate, the plate must be immediately spun to properly mix the sample with the medium. The combined medium is allowed to harden before being incubated at the appropriate temperature to grow the microorganisms contained in the sample.
The number of isolated colonies is counted after incubation. It is advisable to repeat the treatment if the colonies are uncountable or fused, or if the count is greater than 300 CFU/mL or fewer than 20 CFU/mL.
Objectives of Pour Plate Technique
- Isolation of microorganisms from a liquid specimen (or suspension)
- To determine viable microbial load, count colony formation units (CFU) per millilitre.
- To isolate a pure microbial culture from a mixed population
- To isolate microorganisms in distinct colonies for the purpose of studying colony characteristics.
Principle of Pour Plate Method
The pour Plate Method is based on the fact that when an agar medium containing viable bacteria is incubated, each viable microbe multiplies and forms a distinct colony. In this approach, a specified volume of the serially diluted liquid sample, generally 1 mL, is appropriately mixed with roughly 15 mL of specific molten agar medium at 40 - 45°C (less than 50°C) in a Petri plate. The medium is allowed to harden before being incubated for 24 - 48 hours at 37°C. Following incubation, the live microorganisms in the sample will form visible colonies on the medium's surface and within it. The visible colonies may be counted, and the CFU/mL can be estimated using the formula below;
Requirements for Pour Plate Method
1. Liquid Specimen (or suspension of the solid sample)
The sample must be liquid or in suspension for the pour plate. The solid sample must be suspended in a suitable solvent that does not impact microbial growth or react with any medium material.
2. Suitable Solid Culture Media
For the isolation and differentiation of suspected (or particular) bacteria, appropriate culture mediums are utilised. The culture media is a melted solid agar medium at a temperature of 40 - 45°C.
3. Petri Plates
Typically, 10 cm Petri plates are utilised. Before pouring samples or media, they must be sterile and labelled.
4. Test-tubes
During sample preparation, test tubes are required for serial dilution. Sterile tubes are required.
5. Sterile Distilled Water (or Sterile Broth)
For serial dilution, either distilled water or broth can be utilised. They can also be used to dissolve a solid or semi-solid material.
6. Micropipette (or Graduated Pipette)
For measuring the sample during serial dilution and sample inoculation, a micropipette with a volume of 0.1 mL or 1 mL is required.
7. Other Laboratory Facilities
Procedure of Pour Plate Method
The main approach for carrying out the pour plate method is as follows:
1. Arrange all of the needs, put on PPE, sterilise the work area, and set up the laboratory equipment.
2. Sample preparation:
- If the material is liquid, serially dilute it to achieve a microbial load of 20 - 300 CFU/mL. (A prior pilot test may provide an exact value. You can make repeated dilutions up to 10-10 and utilise various dilutions.)
- Dissolve the material in sterile distilled water, sterile broth, or any other solvent if it is solid or semisolid. (Typically, 1 gm of material is combined with 9 mL of solvent to achieve a concentration of 10-1 gm/mL.)
3. Media preparation:
Preparation and autoclaving of appropriate media (general-purpose media such as Nutrient Agar and Plate Count Agar for bacteria, and Potato Dextrose Agar or Sabouraud Dextrose Agar for fungi). Allow the medium to cool to around 40 - 45°C (maximum of 55°C), but do not allow it to harden.
If the medium has previously been prepared and hardened, melt it over a water bath or other heat source.
If you want to mix the sample with media before pouring it into the Petri plate, put around 15 mL of media in a test tube or beaker and autoclave it. Alternatively, a predetermined volume of media can be generated in a large beaker or bottle, and a sample added later by calculating the volume, which will be comparable to 1 mL sample per about 15 mL of media.
4. Arrange sterile Petri plates:
Label the plate's bottom edge with the dilution factor, date, name, sample ID, and other necessary information.
5. Inoculation:
Method – I
- Using a sterile micropipette or calibrated pipette, place 1 ml of diluted sample in the centre of the Petri plate.
- Open the bottle's lid and light the bottle's mouth on fire. Pour around 15 mL of sterilised molten medium over the sample at the proper temperature.
- Close the plate cover and gently spin the plate to adequately mix the sample in the medium. The plate is often swirled in the shape of a "S" or "8."
- (Put sample at specific dilution in the plate labeled with the specific dilution factor.)
Method – II
Add 1 mL of sample to a tube containing roughly 15 mL of molten medium at an appropriate temperature. In the medium, thoroughly mix the sample. Fill a sterile Petri dish halfway with the medium.
6. Replace the Petri plate lid. Allow the media to solidify fully.
7. Invert the plate and incubate it for a reasonable period of time (usually 24 hours at 37°C).
Result Interpretation of Pour Plate Method
- After incubation, formed colonies are observed. One viable microbial cell or colony-forming unit will be present in each colony.
- If all of the colonies are the same kind, we may assume that the sample only included one type of microbial genus. However, various genera or species may produce similar sorts of colonies. As a result, they require an additional test for identification.
- We can deduce that the sample contains a mixed population if the colonies have various morphologies. They can be purified by streak plate sub-culturing each colony on a separate culture medium plate.
- Count the colonies and use the following calculation to determine CFU/mL:
- This will tell you the total number of live microbial cells in the sample.
- The number of colonies must be between 20 and 300 CFU/mL for the best count. If this limit is exceeded, the entire operation must be redone. If the number of colonies is less than 20, it is recommended to use a lower dilution sample on repeated repeats, however if the total number of colonies exceeds 300, it is recommended to use a larger dilution sample on successive rounds.
- If the colonies are merged or the entire plate is covered by a single colony, report "too numerous to count" (TNTC) and restart the sample-taking process at a higher dilution.
Precautions during Pour Plate Technique
1. Adhere to correct safety standards. Consider any unknown or clinical specimen to be dangerous and adhere to safety precautions accordingly.
2. Each Petri plate and medium must be sterile. Sterility is required in the workplace.
3. Sterile water or medium must be used in serial dilution or to dissolve the solid sample.
4. The solvent used to dissolve the solid sample must be sterile and have no growth-promoting or inhibiting effects on microorganisms.
5. The sample must be sufficiently diluted so that the viable microbial load ranges between 20 and 300 CFU/mL. Above this range, counting the colonies becomes difficult, and colonies may merge together. The result is labelled as not significant if it falls outside of this range. If the colony count is less than 20 CFU/mL or greater than 300 CFU/mL, the operation must be repeated.
6. During serial dilution and medium preparation, precise measurement is required.
7. When transferring samples on a Petri plate, medium tube, or bottle, use a micropipette or a standard calibrated tube.
8. The US FDA allows a volume of medium of 12 to 15 mL per 10 cm Petri plate, but the USP allows a level of 15 to 20 mL per 10 cm Petri plate.
9. Before solidifying, the sample must be well mixed with the molten agar.
10. The temperature of the medium during pouring or mixing with the sample is critical. It must be between 40 and 450 degrees Celsius. (550C is the maximum temperature and must not be more than this at any cost). Temperatures below 400°C are not suggested since the medium will either harden or clump.
11. Prior to the addition of medium and samples, each Petri plate must be labelled. The dilution factor should be indicated. While distributing the sample, the labelling must be examined and the dilution factor information must be matched with the sample concentration.
Pour Plate Technique Applications
- Used to identify and count viable bacteria and fungus from suspensions or liquid samples (calculate CFU/ml).
- In the food and pharmaceutical sectors, it is used to isolate microbes and calculate CFUs from raw materials and products like as water, drinks, food, tissue samples, and so on. This will aid in quality control by determining if the product is safe to ingest.
- Used to isolate and count microorganisms in soil in order to understand soil microflora.
- When researching microbial metabolisms and biochemical characteristics, as well as the influence of environmental conditions on microbial development, growth curves are generated.
- Used to obtain discretely separated colonies for pure culture and research into biochemical properties.
- Used to distinguish pure civilizations from mixed cultures.
Advantages of Pour Plate Technique
- It is simple to carry out and does not necessitate the use of any additional instruments or materials for inoculation.
- There is no need for previously solidified agar media.
- There is no risk of gouging during inoculation, as there is with streaking and spreading.
- Even extremely modest levels of microbial burden can be identified.
- Isolated colonies of microorganisms can be obtained in addition to their isolation. The number of CFU/mL can also be determined.
- It may work with any kind of specimen, such as clinical or environmental samples, liquid or solid. (it can be dissolved).
- It may be used to isolate facultative and anaerobic microbes. This procedure may also be used to isolate aerobes.
Limitations of Pour Plate Technique
- Prior to inoculation, solid or semisolid materials must be suspended. If the material is not easily soluble, it is extremely challenging.
- The sample must be serially diluted or else too many colonies will emerge that cannot be counted or recognised as distinct.
- Molten media at 40-45°C can harm heat-sensitive organisms.
- The development of organisms and the establishment of colonies takes time. There will be less oxygen available to microbial cells near the bottom of the solidified medium, causing them to proliferate slowly.
- Colonies may be smaller than in streaking or spreading, increasing the likelihood of missing them.
- Obligate aerobes may struggle to develop towards the bottom of the plates. Some never even grow.
- The procedure takes time since it requires dissolving solid samples, successive dilutions, and melting the medium at a certain temperature (42 - 45°C).



.webp)