A combination of morphological and biochemical tests are required to identify a yeast. Useful morphological characteristics include the colour of the colonies, the size and shape of the cells, the presence of a capsule, the production of hyphae pseudohyphae, and the production of chlamydospores, Useful biochemical tests include the assimilation and fermentation of sugars, and the assimilation of nitrate.
Most yeasts associated with human infections can be identified using one of the commercial test products that are based on sugar assimilation of isolates. However, it is important to remember that morphological examination is essential to avoid confusion between organisms with identical biochemical profiles, in addition there are a number of simple tests for the presumptive identification of some of the most important yeasts, These include the serum germ tube test for the rapid identification of Candida albicans and the urease test for Cryptococcus neoformans.
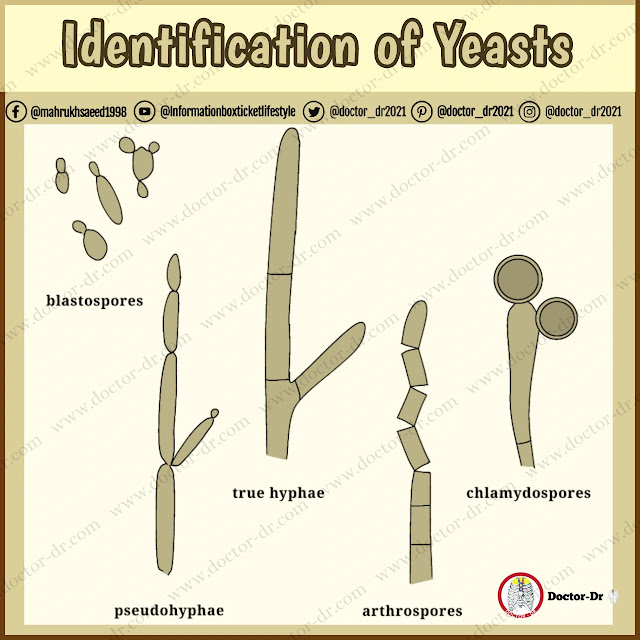
Yeasts are unicellular fungi which consist of round, oval or elongated cells, or blastospores, that propagate by budding out similar cells from their surfaces, The bud may become detached from the parent cell, or remain attached and produce another bud itself. In this way a chain of cells may be produced. The term pseudo hypha is used to describe a chain of yeast cells which have become elongated before budding and have remained attached to one another. Unlike a true hypha, the connection between adjacent pseudo hyphal cells shows a marked constriction. In addition to pseudo hyphae some species of Candida and Trichosporon can produce true hyphae. The hyphae of these organisms, like those of the moulds can fragment to form chains of individual cells, or arthrospores. Although it is an arthrosporic mould, Geotrichum candidum has been included in this chapter because its colonies are similar in appearance to those of Blastoschizomyces capitatus and Trichosporon beigelii.
There are two simple tests that should be performed on yeast isolates:
1. the germ tube test
2. microscopic examination for the presence of a capsule.
If the germ tube test is positive, the organism is Candida albicans, if a capsule is present, this permits the presumptive identification of the organism as Cryptococcus neoformans, but further tests are necessary to confirm it, if germ tubes are not produced and a capsule is absent, further morphological and biochemical tests should be performed.
Germ Tube Test
The germ tube test allows a rapid identification of Candida albícans using either the original isolation plate or a purified culture. The test consists in taking a light loopful of inoculum from a culture plate, suspending it in 0.5 mL of sterile horse serum and incubating at 37oC for 2-3 h. A drop of the suspension is then placed on a microscope slide with a cover slip and the preparation examined under a microscope. The isolate under test is C. albicans if the cells have produced short hyphae and there is no constriction at the junction between the parent cell and the hypha.
This test has some drawbacks. Fewer than 10% of blastospores produce germ tubes in many positive tests; about 5% of Candida albicans isolates fail to produce germ tubes; over-inoculation of the serum can result in inhibition of germ tube formation; and too short an incubation period can lead to false negative results: 2 h is a minimum, not a maximum incubation time, Confusion often arises between C. albicans and C. Tropicalis in the germ tube test, as the yeast cells of the latter tend to produce pseudo hyphae. The distinction is made by observing the constriction between the parent cell and the pseudo hypha in C. tropicalis.
CAPSULE PRODUCTION
Cryptococcus neoformans produces round to oval cells with polysaccharide capsules. These can be detected when the cells are mounted in a pigmented colloidal mounting fluid, such as India ink, which does not penetrate the capsular envelope. The test consists in taking a light loopful of inoculum from the culture and suspending it in a drop of 50% aqueous India ink on a microscope slide. If a capsule is present, it should be visible as a clear halo around the cells, The presence of a capsule gives a presumptive identification of Cr. neoformans but does not provide a definitive identification.
Drawbacks of this test include lack of prominent capsules in some Cr. Neoformans isolates and the possibility of loss of the capsule following subculture of the organism.
UREASE TEST
Urease production is a characteristic of most Cryptococcus neoformans isolates and is useful for their presumptive identification. The test consists in taking a light loopful of inoculum from the original isolation plate, spreading it over the surface of a Christensen's urea slope and incubating at 30oC for up to four days. A colour change from amber to pink permits the presumptive identification of the isolate as Cr. neoformans. However, other species of Cryptococcus, as well as Rhodotorula and Trichosporon species, can give a positive result. Bacterial contamination can also result in a change in the colour of the medium.
MORPHOLOGICAL EXAMINATION
Morphological examination of yeast isolates under the microscope is essential to avoid errors in identification of organisms with identical biochemical profiles. Growth in microaerophilic conditions on cornmeal or other starch-containing media, such as rice agar, stimulates the formation of hyphae, pseudo hyphae, arthrospores and chlamydospores in those species able to produce them.
The surface of the medium (cornmeal agar) is inoculated across the centre of the plate using a wire loop. A sterile cover slip is placed over part of the inoculum and the plate incubated at 30oC for at least 48 h. At intervals, for a period of up to one week, the lid of the plate is removed and the growth under the cover slip examined under the low power objective of a microscope.
Chlamydospores (which can take up to four days to develop on cornmeal agar) are indicative of Candida albicans. Use of Czapek Dox plus Tween 80 medium often stimulates their production within 24 h. If only pseudo hyphae are found, the isolate is a Candida species other than C, albicans. If arthrospores are present, the isolate is a presumptive Trichosporon species. However, it should be noted that C. guilliermondi needs three to four days' incubation to produce pseudo hyphae while T. beigeli needs a similar time to produce arthrospores.
Biochemical Tests
Most laboratories now use commercial identification systems to determine the biochemical profile of yeasts isolates. These kits are less time consuming to set up, simpler to interpret, and often permit more rapid identification of isolates than the classical assimilation and fermentation methods which they have replaced. Some offer extensive databases capable of identifying a wide range of organisms, but others are much more limited in scope. The principal assimilation and fermentation reactions used in comparing organisms are listed in table 1 and 2.
|
|
GLU |
GAL |
SUC |
MAL |
LAC |
RAF |
CEL |
RHA |
TRE |
|
B. capitatus |
+ |
+ |
- |
- |
- |
- |
- |
- |
- |
|
C. albicans |
+ |
+ |
+ |
+ |
- |
- |
- |
- |
+ |
|
C. glabrata |
+ |
- |
- |
- |
- |
- |
- |
- |
+ |
|
C.
guilliermondi |
+ |
+ |
+ |
+ |
- |
+ |
+ |
V |
+ |
|
C. kefyr |
+ |
+ |
+ |
- |
+ |
+ |
V |
- |
V |
|
C. krusei |
+ |
- |
- |
- |
- |
- |
- |
- |
- |
|
C. liplytica |
+ |
- |
- |
- |
- |
- |
- |
- |
- |
|
C.
lusitaniae |
+ |
V |
+ |
+ |
- |
- |
+ |
+ |
+ |
|
C.
parapsilosis |
+ |
+ |
+ |
+ |
- |
- |
- |
- |
+ |
|
C.
pelliculosa |
+ |
V |
+ |
+ |
- |
V |
+ |
- |
+ |
|
C.
tropicalis |
+ |
+ |
V |
+ |
- |
- |
V |
- |
+ |
|
Cr. neoformans |
+ |
+ |
+ |
+ |
- |
+ |
V |
+ |
+ |
|
G. candidum |
+ |
+ |
- |
- |
- |
- |
- |
- |
- |
|
R. glutinis |
+ |
+ |
+ |
+ |
- |
+ |
+ |
V |
+ |
|
S. cerevisiae |
+ |
+ |
+ |
+ |
- |
+ |
- |
- |
V |
|
T. beigelii |
+ |
V |
V |
V |
+ |
V |
V |
V |
V |
|
Key: CEL,
cellobiose; GAL, galactose; GLU, glucose; LAC, lactose; MAL, maltose; RAF,
raffinose; RHA, rhamnose; SUC, sucrose; TRE, trehalose; V, variable reaction |
|||||||||
|
Table 2 Fermentation and other reactions. |
|||||||
|
|
GLU |
SUC |
MAL |
LAC |
TRE |
NIT |
URE |
|
B. capitatus |
- |
- |
- |
- |
- |
- |
- |
|
C. albicans |
+ |
- |
+ |
- |
+ |
- |
- |
|
C. glabrata |
+ |
- |
- |
- |
+ |
- |
- |
|
C. guilliermondi |
+ |
+ |
- |
- |
+ |
- |
- |
|
C. kefyr |
+ |
+ |
- |
+ |
- |
- |
- |
|
C. krusei |
+ |
- |
- |
- |
- |
- |
- |
|
C. liplytica |
- |
- |
- |
- |
- |
- |
+ |
|
C. lusitaniae |
+ |
+ |
V |
- |
+ |
- |
- |
|
C. parapsilosis |
+ |
- |
- |
- |
- |
- |
- |
|
C. pelliculosa |
+ |
+ |
V |
- |
- |
+ |
- |
|
C. tropicalis |
+ |
+ |
+ |
- |
+ |
- |
- |
|
Cr. neoformans |
- |
- |
- |
- |
- |
- |
+ |
|
G. candidum |
- |
- |
- |
- |
- |
- |
- |
|
R. glutinis |
- |
- |
- |
- |
- |
+ |
+ |
|
S. cerevisiae |
+ |
+ |
+ |
- |
V |
- |
- |
|
T. beigelii |
- |
- |
- |
- |
- |
- |
+ |
|
Key: GLU, glucose fermentation; SUC, sucrose fermentation;
MAL, maltose fermentation, LAC, lactose fermentation; TRE, trehalose fermentation;
NIT, nitrate assimilation; URE, urease production; V, variable reaction |
|||||||
Media for Yeast Identification
|
Christensen’s urea agar |
This media is useful for the presumptive
identification of Cryptococcus neoformans. It is important to remember that
other species of Cryptococcus, as well as Rhodotorula and Trichosporon
species, can also give a positive result. |
|
|
Glucose |
1 g |
|
|
Mycological peptone |
1 g |
|
|
Sodium chloride |
5 g |
|
|
Potassium dihydrogen orthophosphate |
2 g |
|
|
Phenol red |
0.012 g |
|
|
Agar |
15 g |
|
|
Distilled water |
1L |
|
|
Heat to dissolve. Autoclave at 115oC for 20 min. Cool
to 50oC and add 50mL of sterile 40% urea solution. |
||
|
Cornmeal agar |
This medium is useful for stimulating the formation
of pseudo hyphae; true hyphae, arthospores an chlamydospores in those species
able to produce them. |
|
|
Cornmeal extract |
2 g |
|
|
Agar |
15 g |
|
|
Distilled water |
1 L |
|
|
Heat to dissolve. Autoclave at 121oC for 15 min. |
||
|
Czapek-Dox plus Tween 80 agar |
This medium is useful for stimulating chlamydospore
production in Candida albicans. |
|
|
Sucrose |
30 g |
|
|
Sodium nitrate |
2 g |
|
|
Potassium chloride |
0.5g |
|
|
Magnesium glycerophosphate |
0.5 g |
|
|
Potassium sulphate |
0.35 g |
|
|
Ferrous sulphate |
0.01 g |
|
|
Agar |
12 g |
|
|
Tween 80 |
10 mL |
|
|
Distilled water |
1 L |
|
|
Heat to dissolve. Autoclave at 121oC for 15 min. |
||
|
Sabouraud’s glucose peptone agar |
This mediumis recommended for the isolation and
cultivation of yeasts. Antibacterial antibiotics (in particular
chloramphenicol) can be added to control bacterial contamination. |
|
|
Glucose |
40 g |
|
|
Mycological peptone |
10 g |
|
|
Agar |
15 g |
|
|
Distilled water |
1 L |
|
|
Heat to dissolve Autoclave at 121oC for 15 min. |
||

%20Technique%20A%20Comprehensive%20Guide%20to%20Water%20Quality%20Testing%20and%20Microbiological%20Analysis.webp)
~1.webp)



.webp)
