Borrelia burgdorferi: A Closer Look at Lyme Disease
Lyme disease, predominantly found in North America and Europe, is caused by Borrelia burgdorferi.- This bacterium belongs to the Spirochaetes phylum, characterized by its spiral or wave-like body and flagella.
- B. burgdorferi is an obligate pathogen, primarily causing infections in humans but can also reside in other mammals as carriers.
- In the lifecycle of ticks, the bacteria are transmitted from infected rodents during larval feeding. These infected ticks then feed on various animals, passing the bacteria to new hosts.
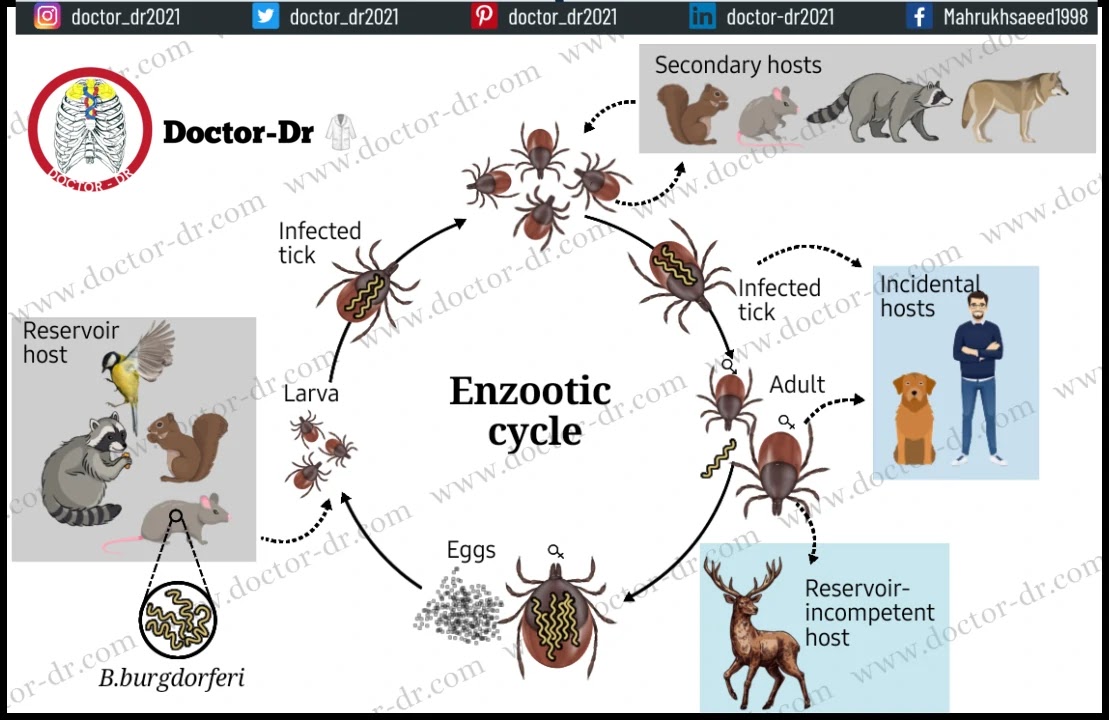
- B. burgdorferi follows an enzootic lifecycle, transferring between Ixodes ticks and vertebrate hosts. While various small mammals can serve as vertebrate hosts, infection only occurs in humans.
- In humans, this leads to Lyme disease, a zoonotic, vector-borne illness transmitted through the tick's saliva.
- The causative agent, B. burgdorferi, is a Gram-negative pathogenic spirochete with a segmented genome consisting of a linear chromosome and multiple linear and circular genomic plasmids.
- Over time, new pathogenic Borrelia species have been discovered, collectively known as B. burgdorferi sensu lato, including species like B. afzelii and B. garinii.
- The genus Borrelia is named after Amedee Borrel, who initially differentiated it from other spirochete types.
- The species name 'burgdorferi' is derived from Willy Burgdorfer, the scientist who first identified this species.
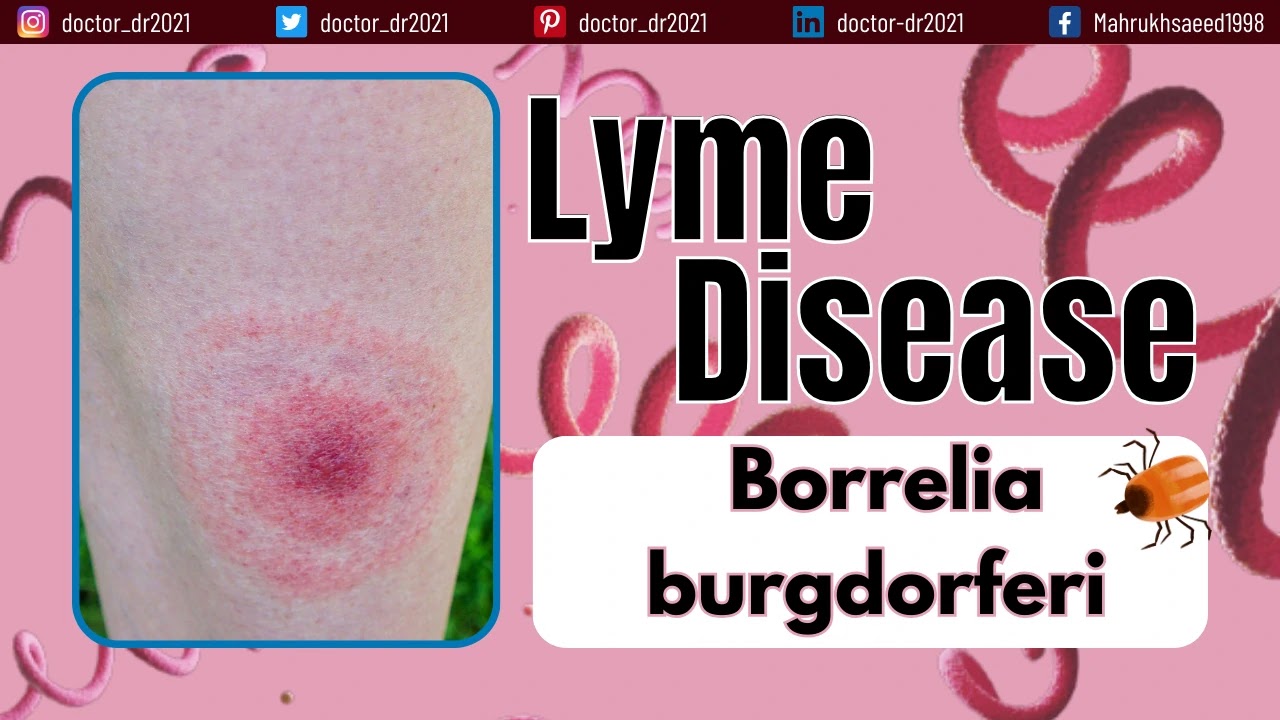
- Lyme disease is characterized by the development of a distinctive red-colored rash resembling a bull's-eye on various parts of the body.
- Lyme disease progresses through three stages, with the severity of infection increasing.
- When the bacteria proliferate in the bloodstream, it can lead to relapsing fever, which may persist for an extended period.
Table of Contents
- Classification of Borrelia burgdorferi
- Habitat of Borrelia burgdorferi
- Morphology of Borrelia burgdorferi
- Cultural characteristics of Borrelia burgdorferi
- Pathogenesis of Lyme disease caused by Borrelia burgdorferi
- Clinical Manifestations of Borrelia burgdorferi
- Laboratory diagnosis of Borrelia burgdorferi
- Treatment of Lyme disease caused by Borrelia burgdorferi
- Prevention and control of Lyme disease caused by Borrelia burgdorferi
Classification of Borrelia burgdorferi
- The genus Borrelia falls within the Spirochaetes phylum, characterized by their helical or spiral-shaped motile cells.
- Borrelia burgdorferi was initially identified as a new species within the Borrelia genus in 1984, based on comparisons with other species in the same genus.
- In recent years, the classification of B. burgdorferi has been refined, taking into account its phenotypic and genotypic characteristics.
- All Borrelia species can be categorized into two primary groups: the first group includes agents responsible for Lyme borreliosis, while the second group comprises species associated with relapsing fever.
- Species belonging to the first group are collectively referred to as B. burgdorferi sensu lato and encompass various species that can cause Lyme disease, such as B. garinii and B. afzelii. The term B. burgdorferi sensu stricto is specifically used to denote B. burgdorferi itself.
- Among the species within the B. burgdorferi group, not all are evenly distributed worldwide, with only three known to cause infections in humans.
- The genetic diversity observed within these species is a result of various molecular mechanisms, including mutations, deletions, and substitutions, which regulate the rate of genetic variability.
The following is the taxonomical classification of B. burgdorferi:
|
Domain |
Eubacteria |
|
Phylum |
Spirochaetes |
|
Order |
Spirochaetales |
|
Family |
Spirochaetaceae |
|
Genus |
Borrelia |
|
Species |
B. burgdorferi |
Habitat of Borrelia burgdorferi
- Borrelia burgdorferi is most commonly found in temperate regions of the northern hemisphere, including countries in North America and Europe.
- This bacterium has the capability to infect a wide range of vertebrate animals, including small mammals, lizards, and birds.
- The lifecycle of B. burgdorferi is zoonotic and involves two distinct hosts: insects (ticks) and vertebrate hosts, including humans.
- The geographical distribution of the bacterium is a result of the overlapping ranges of competent reservoir hosts for B. burgdorferi and the tick vectors.
- In the northern hemisphere, the primary tick species responsible for human disease transmission is Ixodes scapularis, while in the western states, it is I. pacificus.
- The role of B. burgdorferi reservoirs in the natural environment remains relatively unexplored, but factors such as local and temporal variations in tick densities play a crucial role in infections.
- The circulation of B. burgdorferi in nature involves a transmission cycle from the reservoir host to feeding tick larvae as part of the bacterium's life cycle.
- These feeding larvae then molt into nymphs, which subsequently feed on a wide variety of animals, including rodents.
- These animals then become new reservoirs, perpetuating the lifecycle. The adult tick stage predominantly feeds on larger mammals.
- The different environments of mammalian and tick hosts necessitate adaptations by the bacterium to thrive in these contrasting conditions.
- The ability to survive in diverse environments is attributed to variations in gene expression, resulting in distinct protein components and physiological adaptations.
- An ecological interplay exists between the tick and B. burgdorferi, influenced by factors such as host movement, availability, species composition, general vegetation characteristics, and microclimate.
Morphology of Borrelia burgdorferi
- B. burgdorferi cells exhibit a flexible helical shape, with dimensions ranging from 0.2-0.3 µm in width and 4-30 µm in length.
- These bacteria are largely pleomorphic, meaning they can alter their morphology in response to environmental conditions.
- Peritrichous flagella adorn the entire cell, serving both as structures for motility and providing structural support. Cell movement is achieved through both rotational and translational motions facilitated by these flagella.
- Each cell possesses between seven to eleven periplasmic flagella located at both ends, with overlapping regions in the central part of the cell.
- B. burgdorferi features a multilayered outer membrane enveloping the protoplasmic cylinder, which is covered by two lipid membranes.
- Beneath the outer membrane lies the cytoplasmic membrane and the enclosed cytoplasmic contents.
- A periplasmic space exists between the outer and inner membranes, containing a peptidoglycan layer and flagella filaments.
- The cellular envelope of B. burgdorferi is distinct from that of typical Gram-negative bacteria, as it lacks lipopolysaccharides found in typical Gram-negative cell walls. Instead, it contains immunoreactive glycolipids.
- An essential component of B. burgdorferi is its outer surface proteins, which play a crucial role in bacterial transmission.
- Interestingly, these bacteria lack genes encoding enzymes required for the biosynthesis of various amino acids, fatty acids, and nucleotides.
- The genome of B. burgdorferi comprises a small linear chromosome of approximately 1000 kb, with a G+C content of 28.6%. It also contains linear and circular plasmids that vary in number and size.
Cultural characteristics of Borrelia burgdorferi
- Borrelia burgdorferi is a fastidious, microaerophilic bacterium that thrives better in liquid media than on solid agar-based media.
- Its growth is reliant on complex nutritional requirements due to its limited biosynthetic capabilities.
- The culture medium used for cultivating B. burgdorferi typically comprises components such as serum, glucose, albumin, peptides, amino acids, and vitamins.
- Additionally, N-acetylglucosamine and both long-chain saturated and unsaturated fatty acids are essential nutritional components.
- B. burgdorferi grows slowly, with cell division occurring every 8-12 hours during the exponential growth phase.
- Typically, the cell density reaches approximately 107-108 cells per ml after 5-7 days of cultivation.
- Isolated colonies of B. burgdorferi can be obtained on semi-solid agar media like Barbour-Stornner-Kelly II (BSK II) or MKP medium. These media are made selective by incorporating antibiotics such as kanamycin, rifampicin, and nalidixic acid as selective agents.
- The colonies are subsurface and lack distinct morphologies, making it challenging to identify the bacteria based solely on growth conditions and colony characteristics.
- B. burgdorferi, classified as a lactic acid bacterium, primarily utilizes carbohydrates, especially glucose, as its major energy source to produce lactic acid.
- The optimal temperature for bacterial growth and fermentation processes falls within the range of 30-34°C, with maximum growth occurring at 33°C.
- The generation time of B. burgdorferi is influenced by various factors, including culture conditions and available nutrients, with a time frame spanning from 7 to 20 hours.
- The following are some cultural characteristics of B. burgdorferi on different culture media:
Borrelia burgdorferi on BSK medium
- Colonies of B. burgdorferi become visible on culture media approximately two weeks after incubation, although a more accurate enumeration and evaluation of colony morphology may require three to four weeks.
- Generally, these colonies appear as small, white discs, but microscopic examination may reveal subtle differences in their morphology.
- These colonies are typically small, compact, and round, with an average diameter ranging from 0.4-0.5 mm, mostly concentrated at the surface of the medium.
- Some larger, more diffuse colonies may also be observed, with an average diameter of 0.5-0.7 mm.
- The colony surface is characterized by intricate networks of coiled spirochetes at the periphery, along with numerous spherical cells.
- Smaller colonies exhibit well-defined, sharp edges, but there are no isolated free spirochetes present on the surrounding agar surface.
- In contrast, diffuse colonies have fewer spherical bodies and a less densely packed appearance.
Biochemical Characteristics
The biochemical properties of B. burgdorferi have not been thoroughly investigated because of the bacterium's challenging growth requirements and fastidious nature.
Pathogenesis of Lyme disease caused by Borrelia burgdorferi
- Lyme disease is caused by the spiral-shaped bacterium B. burgdorferi, which is transmitted to humans through the bite of small ticks belonging to the Ixodes genus.
- Humans are considered "accidental" hosts for B. burgdorferi because the spirochetes from infected humans do not typically transmit the infection to other hosts.
- While residing in the midgut of Ixodes ticks during periods between feedings, B. burgdorferi spirochetes enter a dormant, non-replicating state where they attach to epithelial cells.
- Here, they express a plasmid-encoded, major outer surface protein known as OspA. This protein serves a critical role in protecting B. burgdorferi from antibodies present in the blood meal of immune hosts and may facilitate colonization of the tick midgut by binding to cell receptors.
- Outer surface protein A (OspA) is specifically expressed on the surface of B. burgdorferi within the unfed tick's midgut, where it binds to gut proteins.
- Upon the tick's feeding, the expression of OspA is suppressed, allowing the spirochetes to migrate to the tick's salivary glands. Concurrently, the expression of outer surface protein C (OspC) is up-regulated, which is crucial for transmission from ticks to mammals.
- In humans, transmission of the pathogen occurs through the bite of an infected tick, introducing the bacterium into the healthy skin.
- This extracellular pathogen initially establishes itself in the dermal tissue, where it adapts to life within the mammalian host by altering the expression of its surface glycoproteins.
- The shift in gene expression patterns in response to tick feeding, particularly the switch from OspA to OspC, is pivotal.
- B. burgdorferi's capacity to multiply and establish infection within the mammalian host's skin is evident in one of the hallmark signs of localized infection, known as stage 1 Lyme disease in humans—the erythema migrans (EM) rash. This rash results from the infiltration of lymphocytes and macrophages.
- Spirochetes are highly motile and are likely coated with the host protease plasmin.
- After a certain period, they can spread through the skin, leading to an enlargement of the rash, often characterized by a central pale area, giving it a "bull's-eye" appearance.
- B. burgdorferi expresses outer surface proteins that specifically engage with endothelial cells, platelets, chondrocytes, and the extracellular matrix through specific interactions with integrins, glycosaminoglycans, fibronectin, and collagen.
- These interactions are crucial for the bacterium's ability to home in on and colonize various tissues, including the skin, joints, and heart. Moreover, the bacterium triggers the activation of proteases and other host cell molecules to facilitate its dissemination through the bloodstream and into different tissues.
- Disseminated infection, also known as stage 2 Lyme disease, involves temporary colonization of the bloodstream.
- During this stage, some degree of vascular damage, such as mild vasculitis or hypercellular vascular occlusion, can be observed in multiple locations, suggesting that the spirochetes colonize the walls of blood vessels.
- In late-stage or stage 3 Lyme disease, bacterial multiplication appears to be significantly reduced or controlled by the host's defenses, resulting in very low bacterial counts in tissues.
- The host's response to B. burgdorferi plays a critical role in the pathogenesis of the disease.
- B. burgdorferi does not produce toxins or proteases directly responsible for tissue damage upon colonization.
- Instead, the bacterium generates various molecules that activate host responses, potentially leading to localized and widespread inflammatory reactions.
- Many of these host responses are typically part of the innate defense and inflammatory response and serve to contain or eliminate infections.
- However, B. burgdorferi can persist despite the development of a robust host immune response once the infection is established in humans. The bacterium can survive for extended periods, even years.
- B. burgdorferi has the capability to bind to mammalian complement regulatory factors, which may confer resistance to complement-mediated lysis and opsonization, aiding in its persistence within host tissues.
Clinical Manifestations of Borrelia burgdorferi
Lyme disease
- Lyme disease is typically classified into three distinct stages.
- The first stage is marked by the appearance of erythema migrans (EM), a characteristic red, ring-shaped skin lesion with a central clearing. It initially develops at the site of the tick bite but can also manifest at remote locations.
- Clinical symptoms during this stage commonly include headaches, fever, muscle and joint pain, as well as a general sense of malaise.
- The second stage, which commences several weeks to months after infection, may encompass symptoms such as arthritis. However, the most significant features involve neurological disorders, which can encompass meningitis, neurological deficits, and carditis.
- This stage results from the hematogenous spread of spirochetes to various organs and tissues.
- Furthermore, neurologic symptoms and infection may affect the meninges, spinal cord, peripheral nerves, and brain.
- The third stage is typically characterized by chronic arthritis or acrodermatitis chronica atrophicans (ACA), which is a diffuse skin rash. This stage can persist for an extended period, even lasting for years.
Laboratory diagnosis of Borrelia burgdorferi
Specimen
- Specimens for testing in Lyme disease cases include blood, cerebrospinal fluid, joint fluid, and tissue biopsies.
- It is important to transport body fluids without the use of preservatives.
- When handling tissue biopsy specimens, they should be submerged in sterile saline to prevent desiccation.
Direct detection methods
- The organisms can be directly observed in wet preparations of peripheral blood when it's mixed with an equal amount of sterile, non-bacteriostatic saline. Under either dark- or brightfield illumination, these spirochetes display rapid movement, often causing red blood cells to shift.
- In the case of Lyme disease, tissue sections are examined using the Warthin-Starry silver stain.
- PCR (Polymerase Chain Reaction) has been instrumental in detecting B. burgdorferi DNA in clinical specimens from patients exhibiting both early and late clinical manifestations.
- Ideal specimens for this purpose include urine, synovial tissue, synovial fluid, and skin biopsies taken from patients with erythema migrans (EM).
Culture
- Culturing B. burgdorferi is typically avoided as it is a time-consuming process, taking 6-8 weeks to complete, and is not very sensitive.
- When performed, culturing B. burgdorferi from specimens using Barbour-Stonner-Kelly medium can provide a conclusive diagnosis.
- Positive cultures have been achieved primarily during the early stages of the illness, notably from biopsy samples of erythema migrans (EM) lesions.
Serology
- Serological testing has traditionally been the primary method for diagnosing Lyme disease. However, it's important to note that 3-5% of both healthy individuals and those with other medical conditions (such as rheumatoid arthritis and various infectious diseases) may initially test positive on serological assays like the enzyme immunoassay (EIA) or indirect fluorescent antibody (IFA) test.
- While an indirect immunofluorescence test is available, enzyme-linked immunosorbent assay (ELISA) has become more widely adopted.
- To confirm serological findings, immunoblotting is employed, utilizing a carefully selected panel of recombinant antigens.
- The interpretation of immunoblots relies on the quantity and molecular size of antibody reactions with B. burgdorferi.
- Immunoblots can be analyzed for both IgG and IgM.
- It is essential to interpret the antigen-antibody band patterns on immunoblots while considering known results from patients at various stages of Lyme borreliosis.
Treatment of Lyme disease caused by Borrelia burgdorferi
- For the early stages of Lyme disease, the most commonly recommended treatment is doxycycline.
- In cases where arthritic symptoms have already manifested, more extended antibiotic courses, such as ceftriaxone, are typically employed.
- When dealing with children and pregnant women, amoxicillin is the preferred antibiotic.
- In the initial stage of Lyme disease, doxycycline, amoxicillin, or cefuroxime, as well as parenteral cephalosporins, are the drugs of choice.
- For patients who do not respond to initial treatment or are in later stages of the disease, broad-spectrum cephalosporins, particularly ceftriaxone or cefotaxime, have been used successfully.
Prevention and control of Lyme disease caused by Borrelia burgdorferi
- A vaccine featuring recombinant outer surface protein A has been approved for human use as protection against Lyme disease resulting from infections by organisms within the B. burgdorferi complex.
- Preventing infection involves measures such as avoiding areas known to have ticks, wearing protective clothing, regularly inspecting clothing, the body, and pets for ticks, and promptly removing them when found.
- The key to prevention is minimizing exposure to ticks.
- Recommendations include wearing long-sleeved clothing and pants, with pants tucked into socks for added protection.
- After outdoor activities, thorough skin examinations can help identify ticks for removal before they have a chance to transmit B. burgdorferi.
- Efforts to control tick populations in the environment through the application of insecticides have shown some success in reducing the number of nymphal ticks during a season.
- Additional preventive measures encompass using insect repellents and wearing clothing that effectively shields the body from tick bites.




~1.webp)
.webp)
.webp)

