The paramyxoviruses include the zoonotic Nipah virus (NiV), which is transmitted by bats.
In Malaysia, it was originally discovered in 1998. Since 1999, however, no instances have been found in Malaysia.
Numerous epidemics in South and Southeast Asia are brought on by the virus.
The primary reservoir for the infection from which illnesses in people and pigs can spread is Pteropus fruit bats.
The Malaysia-Singapore outbreak of the virus was associated with contact with pigs whereas the Indo-Bangladesh outbreak was associated with the consumption of raw date palm sap contaminated by fruit bats.
The virus, which is extremely contagious and spreads through infected people and animals, causes severe neurological and respiratory illness.
The virus is categorised as a Biosafety Level 4 (BSL 4) pathogen because of its high mortality rate, rapid rate of transmission, and lack of an effective antiviral drug or vaccine.
Table of Contents
- Structure of Nipah Virus (NiV)
- Genome structure of Nipah Virus (NiV)
- Epidemiology of Nipah Virus (NiV)
- Transmission of Nipah Virus (NiV)
- Replication of Nipah Virus (NiV)
- Pathogenesis of Nipah Virus (NiV)
- Clinical Manifestations of Nipah Virus (NiV)
- Diagnosis of Nipah Virus (NiV)
- Treatment of NiV
- Prevention and Control of NiV
Structure of Nipah Virus (NiV)
- The Paramyxoviridae family of enveloped viruses includes the Nipah virus.
- It has a helical nucleocapsid and a negative-sense, single-stranded, non-segmented RNA genome.
- The viral ribonucleoprotein is made up of the long polymerase, phosphoprotein, and nucleocapsid.
- The matrix protein that surrounds the nucleocapsid is embedded with spike-like fusion proteins and glycoproteins that are in charge of cellular adhesion and host cell entrance.
- The NiV range in size from 40 to 1900nm, making them bigger than ordinary paramyxoviruses on average.
- The presence of reticular cytoplasmic inclusions near to the endoplasmic reticulum distinguishes the virus from other paramyxoviruses.
- The virus closely resembles the Hendra virus (HeV), differing just slightly in its ultrastructure, and exhibits strong cross-reactivity in a number of serological assays.
- The two strains, Bangladesh (BD) and Malaysian (MY), vary in their virulence and mode of transmission yet have a 92% sequence similarity.
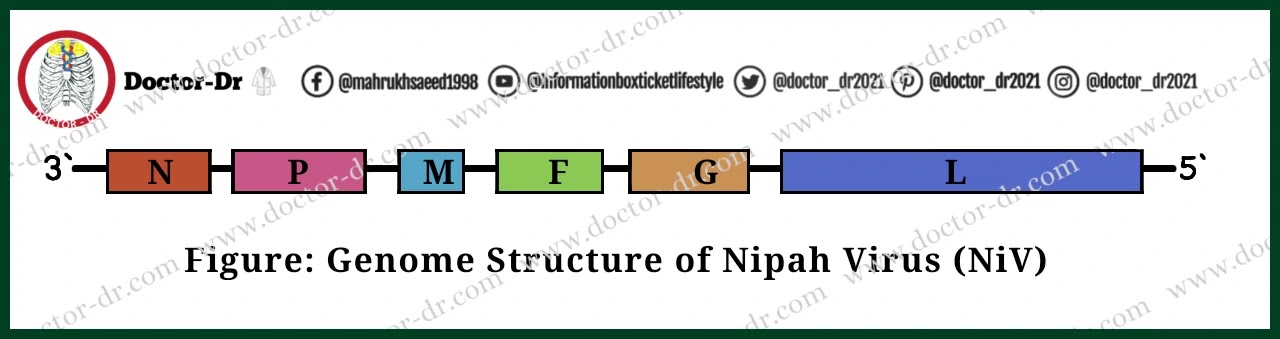
Genome structure of Nipah Virus (NiV)
- A negative-sense, single-stranded, non-segmented RNA with a genome size of around 18.2 kbp makes up the NiV's genome.
- Nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), attachment glycoprotein (G), and the large protein or RNA polymerase protein (L) are the six structural proteins that it codes for.
- Three additional nonstructural proteins are also encoded by the P gene either by RNA editing (V and W proteins) or an alternative open reading frame (C protein).
Epidemiology of Nipah Virus (NiV)
- In Malaysia and Singapore, the first Nipah virus outbreak was documented between 1998 and 1999.
- The diagnosis of severe viral encephalitis in 94 people who had intimate contact with the swine population between September 1998 and June 1999 demonstrated the virus's direct transfer from pigs to humans.
- Between 1998 and 1999, 246 patients in Singapore and Malaysia were diagnosed with febrile encephalitis caused by NiV. The mortality rate for people was about 40%.
- In 2001, a geographically disjointed area—the Meherpur region of Bangladesh and Siliguri city of West Bengal, India—reported the second epidemic.
- In all, 9 NiV outbreaks were documented in Bangladesh up until the year 2010.
- A further epidemic in Bangladesh in 2011 was reported to have resulted in a total of 15 fatalities from infection with the Nipah virus.
- Epidemiological studies revealed that the NiV virus was spreading by zoonotic and human-to-human transmissions throughout Asia, Africa, and the South Pacific Ocean.
- Over the last 20 years, it has caused the lives of hundreds of people and constituted a serious threat to both people and domestic animals.
Transmission of Nipah Virus (NiV)
The NiV can transmit to people from:
- Direct contact with sick animals, such as pigs or bats, or their bodily fluids, such as blood, urine, saliva, etc.
- Consumption of tainted food items that have been polluted by diseased animals (such as palm sap or fruit that has been tainted by fruit bats)
- Close contact with a NiV-positive individual or their bodily fluids (such as blood, urine, nasal or respiratory droplets)
The intake of fruits and fruit products (such raw date palm juice) contaminated with the saliva from the infected fruit bats was discovered to be the most likely cause of infection during the epidemics that followed in Bangladesh and India.
The NiV virus was shown to directly transfer from person to person during later epidemics in Bangladesh and India through close contact as well as bodily fluids and secretions.
Numerous family members and carers of the afflicted individuals also reported human-to-human transfers.
Replication of Nipah Virus (NiV)
Attachment/Adsorption
The viral glycoprotein G and the B2 receptor help the virus bind to its host cell, which is then followed by the fusion of the viral and host membranes.
Penetration and Biosynthesis
The transcription of viral mRNA, which is then translated into viral proteins, uses the genome of the negative RNA as a template. Additionally, the synthesis of cRNA(+) uses the vRNA as a template, and cRNA(+) in turn uses the vRNA as a template. Additionally, the viral proteins contribute to interferon signalling. Additionally, the cells endocytose and mature the F proteins.
Assembly
N, P, C, M, F, and G are all included in the virions during assembly, which is predominantly carried out by the M protein.
Release
The virions are expelled from the cell after budging through the host membrane, which has G proteins on its surface.
Pathogenesis of Nipah Virus (NiV)
- In the initial stages of NiV infection, the virus can be observed in the epithelial cells of the bronchioles.
- NiV antigen can be identified in both the bronchi and alveoli.
- Infection of the airway epithelium triggers the activation of inflammatory mediators.
- As the disease progresses, the virus spreads to the endothelial cells of the lungs.
- The virus enters the bloodstream, disseminating either freely or bound to host leukocytes, ultimately reaching the brain, spleen, and kidneys.
- Two distinct pathways facilitate viral entry into the central nervous system (CNS) – one via the hematogenous route and the other anterogradely via olfactory nerves.
- Infection of the CNS disrupts the blood-brain barrier (BBB), leading to the expression of IL-1β and tumor necrosis factor (TNF)-α, resulting in the development of neurological symptoms, as indicated in red font for human symptoms.
- Through the oro-nasal pathway, the NiV infects the host and spreads.
- It is still unclear exactly where human first replication occurs.
- However, because of the large amounts of antigen present there after infection, the lymphoid and pulmonary tissues are regarded as potential first replication sites.
- Acute respiratory distress syndrome (ARDS)-like condition can be brought on by the viral antigens that can be found in the bronchi and alveoli. These cytokines cause inflammation.
- The virus subsequently spreads to the lungs' endothelial cells in the latter stages of the illness.
- The virus spreads as a result of the early viremia, and then the endothelium becomes the site of secondary replication.
- The Ephrin-B2 receptor on the endothelium, as well as the lungs, placenta, prostate, blood vessels, and several other organs, is where the viral glycoprotein G binds.
- Ephrin-B2 is highly conserved across multiple animal groups, with similarities between bats and pigs of about 95–96%, which helps to explain the great variety of hosts connected to the Nipah virus.
- The virus enters the brain either retrogradely through the olfactory neurons or hematogenously through the bloodstream.
- It produces IL-1 and tumour necrosis factor (TNF), which cause neurological symptoms throughout the infection. It also disturbs the blood-brain barrier (BBB).
- Because the virus avoids the innate immune response, it is very deadly.
- Interferon activity is also inhibited by the P gene products.
- The various NiV virus strains are thought to have coevolved independently with their reservoirs, which accounts for the variations in virulence and epidemiological characteristics.
Clinical Manifestations of Nipah Virus (NiV)
Infection with the Nipah virus can result in mild to severe symptoms, including encephalitis and eventual death.
The virus typically takes 4 to 14 days to fully incubate. However, a 45-day incubation time has also been recorded as being prolonged. General signs of infection throughout the early stages include:
- Fever
- Headache
- Cough
- Sore throat
- Difficulty in breathing
- Myalgia
- Vomiting
Severe symptoms might then develop, and within 24 to 48 hours, a coma could develop. These signs might be:
- Disorientation
- Drowsiness
- Confusion
- Seizures
- Coma
- Brain swelling (encephalitis)
Patients who have aberrant doll's eye reflexes, pupillary reflexes, vasomotor abnormalities, seizures, and myoclonic jerks are said to have brainstem dysfunction.
Relapse or late-onset encephalitis, some of which can happen months or years after the initial infection, are characteristics of the NiV infection.
Some individuals also have psychological symptoms such sadness, personality problems, trouble concentrating, and/or memory loss.
During the outbreaks in Malaysia and India, differences in clinical symptoms were also seen between the various strains.
In contrast to the outbreak in Malaysia, where no significant respiratory involvement was seen and had a relatively lower mortality rate of 40%, respiratory illness like cough, respiratory distress, and atypical pneumonia were observed in 70% of patients there. This outbreak in India and Bangladesh also had a higher mortality rate of 70%.
Diagnosis of Nipah Virus (NiV)
The samples for the serological testing, which may include throat swabs, urine, blood, and/or CSF for diagnosis, should be obtained 10–14 days after the beginning.
A BSL-4 laboratory should process the sample.
However, following viral inactivation by sample irradiation, it may also be handled in a BSL-2 lab.
PCR
Real-Time PCR (RT-PCR) can be used to identify NiV RNA in respiratory secretions, urine, or CSF. High specificity and sensitivity characterise this method. The SYBR Green-based test also detects the HeV but only identifies a different portion of the N gene, whereas the TaqMan probe-based assay detects the N gene and has a better sensitivity.
Immunohistochemistry
Immunohistochemistry may be performed on a broad variety of formalin-fixed tissues from many organs, including the brain, lung, spleen, kidney, and lymph nodes. Previously, convalescent human serum was utilised for immunohistochemistry; presently, NiV-specific rabbit serum is used instead.
Virus Isolation
The Vero cell line is the preferred cell line for the viral isolation from the samples, which must be carried out in a BSL-4 facility. Within three days, cytotoxic effects are visible, and NiV-formed syncytia, which are bigger than those produced by HeV and aid in differentiating the two, are seen.
ELISA
It is the most typical serological technique for finding NiV infection. IgG and IgM are both detected with it. On the first day of the illness, 50% of patients have IgM antibodies detectable, whereas 100% of patients have IgG antibodies positive. IgG lasts for a number of months.
Serum Neutralization Test
Although it needs to be carried out in a BSL-4 laboratory, it is regarded as the most accurate test for detecting NiV infection. The test sera are used to infect the Vero cells, which are then exposed to the virus for three days while being watched for cytopathic effects.
Treatment of NiV
- The NiV infection can only be managed by supportive care and acute encephalitis treatments.
- Anticonvulsant medication, secondary infection care, mechanical breathing, and rehabilitation are some examples of supportive care.
- For the potential therapy of NiV, three pharmaceuticals have been investigated: ribavirin, m102.4 monoclonal antibodies, and favipiravir.
Prevention and Control of NiV
- An effective vaccine candidate against NiV involves the use of a recombinant measles virus (rMV) vaccine, engineered to express the envelope glycoprotein of NiV.
- In recent years, a recombinant vaccine has been developed using a replication-competent vesicular stomatitis virus, which encodes a NiV glycoprotein.
- Vaccine research has yielded Nipah virus-like particles (NiV-VLPs), composed of three NiV proteins (G, F, and M) produced from mammalian cells. These NiV-VLPs have been successfully validated as vaccines in BALB/c mice.
- Cutting-edge immunoinformatics techniques have been harnessed to create a peptide-based NiV vaccine. This innovative approach involves the prediction and modeling of T-cell epitopes from NiV antigenic proteins.
The NiV virus has few effective therapies, thus illness prevention must be given first emphasis.
Despite the high mortality rates of one of the WHO's priority infections, there are still no licenced and effective vaccines for the NiV infection in humans. However, Public Health Vaccines, the University of Tokyo, and the University of Oxford are testing Nipah virus vaccines in preclinical studies with the intention of testing their safety and efficacy in the next years.
Among the steps used to avoid the illness are:
- Interventions to stop the spread of the virus to agricultural animals by keeping them from consuming fruit bat-contaminated with the virus
- Farms shouldn't be located next to fruit trees that draw bats.
- Consuming tainted sap must be prevented.
- installation of physical obstacles to stop bats from climbing trees
- The specimens must be handled with minimal aerosol generation techniques in appropriate biosafety cabinets.
- Patients or farm animals suspected of having the NiV virus need to be separated right away.
- Hospitals and healthcare facilities must be ready to screen patients and handle any outbreaks in disease-prone areas.
- Proper handwashing and hygiene practice must be exercised before and after handling specimens or contact with the patient.


.webp)
.webp)


~1.webp)
.webp)
.webp)

