Hemoglobin is a sophisticated iron-rich protein present in the erythrocytes (red blood cells) of the majority of vertebrates, where it plays a critical role in the transportation of oxygen and carbon dioxide in the bloodstream.
Furthermore, it is the factor responsible for the crimson coloration of our blood. Hemoglobin is present in animals with red-hued blood, encompassing nearly all vertebrates with the exception of Channichthyidae family fish and a limited number of invertebrates.
Hemoglobin is abbreviated as Hb or Hgb.
- In addition to red blood cells, hemoglobin is also present in alveolar cells, macrophages, select neurons in the midbrain, mesangial cells in the kidneys, hepatocytes, vaginal epithelial cells, cervical cells, and more.
- Hemoglobin is produced concurrently with red blood cells (RBCs) during the process of erythropoiesis in the bone marrow. The iron component (heme) is synthesized within the cytoplasm and mitochondria of developing RBCs, while the protein part (globin protein) is manufactured by the ribosomes within the maturing RBCs. The production of globin is regulated by three distinct genes: the alpha-globin genes HBA1 and HBA2, and the beta-globin gene HBB.
- Hemoglobin (Hgb) is the primary constituent in blood responsible for transporting oxygen and carbon dioxide gases. Each Hgb molecule can bind with up to four molecules of oxygen or carbon dioxide simultaneously. These gases attach to the heme component of hemoglobin. When oxygen combines with hemoglobin, it forms oxyhemoglobin, which carries approximately 98% of the oxygen in our blood. Similarly, when carbon dioxide binds with hemoglobin, it creates carbaminohemoglobin, which transports about 25% of the released carbon dioxide in the bloodstream.
Table of Contents
- Structure of Hemoglobin
- Types of Hemoglobin
- Functions of Hemoglobin
- Normal Hemoglobin Level
- Diseases Related to Hemoglobin
Structure of Hemoglobin
- Hemoglobin (Hgb) is a globular metalloprotein characterized by a quaternary structure. A single hemoglobin molecule consists of four subunits, each featuring a polypeptide chain (globin protein) linked to a prosthetic heme group. Each subunit has a molecular weight of approximately 16,000 Daltons, resulting in a total molecular weight of 64,000 Daltons for the Hgb molecule.
- In adults, the polypeptide chains come in two varieties: the alpha chain and the beta chain, with 141 and 146 amino acids, respectively. Adult hemoglobin comprises two alpha subunits (α1 and α2) and two beta subunits (β1 and β2), forming two αβ dimers that arrange around a 2-fold axis of symmetry. Each subunit features a heme group attached to the globin protein. Fetal hemoglobin substitutes the beta subunits with gamma subunits (γ1 and γ2). In a rare form of hemoglobin, the beta subunits are replaced by delta subunits (δ1 and δ2).
- The heme group encompasses an iron atom (ferrous ion, Fe+2) situated at the center of a porphyrin ring, binding to the ring's nitrogen atoms. The Fe+2 ion is linked to the globin subunit via a histidine residue within a pocket. Each Fe+2 ion can bind to either one oxygen (O2) molecule or one carbon dioxide (CO2) molecule.
Types of Hemoglobin
Considering the non-alpha subunits, normal hemoglobin primarily exists in three distinct types:
1. Haemoglobin A
Constituting approximately 95 to 98% of the total adult hemoglobin, this variant features two alpha subunits and two beta subunits.
2. Hemoglobin A2
Comprising approximately 2 to 3% of the total adult hemoglobin, it consists of two alpha subunits and two gamma subunits.
3. Hemoglobin F
This hemoglobin type is characteristic of fetuses and newborns, with minimal representation, less than 1%, in adults. It comprises two alpha subunits and two delta subunits.
In addition to these primary hemoglobin types, there exist other mutated variants, such as hemoglobin E, hemoglobin S, and hemoglobin C.
Functions of Hemoglobin
Hemoglobin's chief role is the transport of oxygen and carbon dioxide gases, while it also plays a secondary role in regulating blood pH and acting as a buffer.
1. Oxygen Transport
The Fe+2 ion within a heme group can attach to a single O2 molecule, enabling one Hgb to transport a total of four O2 molecules. When O2 is linked with Hgb, it is referred to as oxyhemoglobin, whereas Hgb lacking bound O2 is termed deoxyhemoglobin. Approximately 98% of the oxygen in the bloodstream is conveyed by oxyhemoglobin.
2. Carbon dioxide Transport
The Fe+2 ion found in a heme group can attach to a single CO2 molecule, enabling one Hgb to transport a total of four CO2 molecules. When CO2 is bound to Hgb, it is referred to as carbaminohemoglobin. Approximately 20 to 25% (with an average of 23%) of the CO2 in the blood is conveyed by carbaminohemoglobin.
3. Other Gases/Ion Transport
In addition to O2 and CO2, Hgb is capable of binding to various other ligands, including carbon monoxide (CO), nitric oxide (NO), sulfur monoxide (SO), nitrite ion (NO-2), sulfides (S-2), and more. Notably, Hgb exhibits an affinity for CO that is over 200 times greater than its affinity for O2. When CO is attached to Hgb, it is referred to as carboxyhemoglobin.
4. Regulation of Blood pH and Buffering Function
Hgb molecules can also interact with hydrogen ions to help regulate the blood's pH.
Normal Hemoglobin Level
Hemoglobin concentration in the blood is typically measured in grams per deciliter (g/dl). The quantity of hemoglobin present varies depending on an individual's age, gender, and overall health. Generally, human hemoglobin levels fall within the range of 12 to 20+ g/dl.
|
Age of Person |
Normal Hgb Level (g/dl) |
|
Newborn |
14 to 24 |
|
2 weeks |
13 to 20 |
|
3 months |
9.5 to 14.5 |
|
6 months to 6 years |
10.5 to 14.0 |
|
6 years to 12 years |
11 to 16 |
|
Adult male |
14 to 18 |
|
Adult female |
12 to 16 |
Diseases Related to Hemoglobin
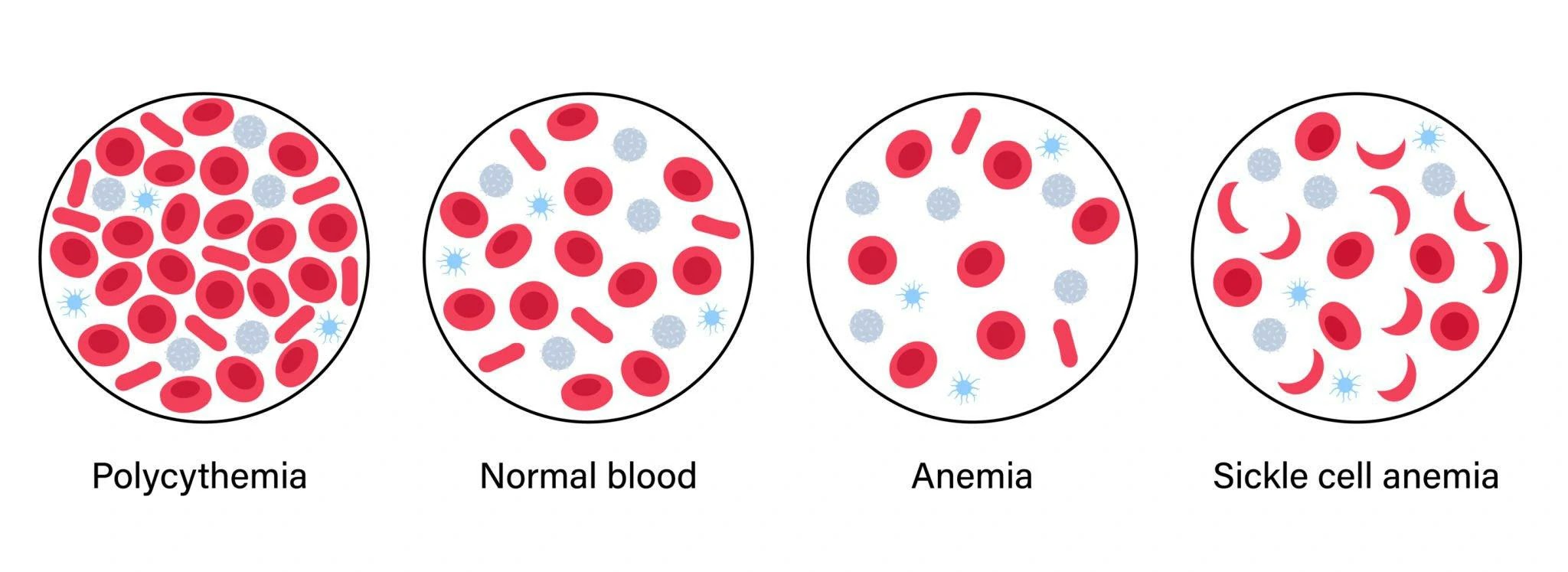
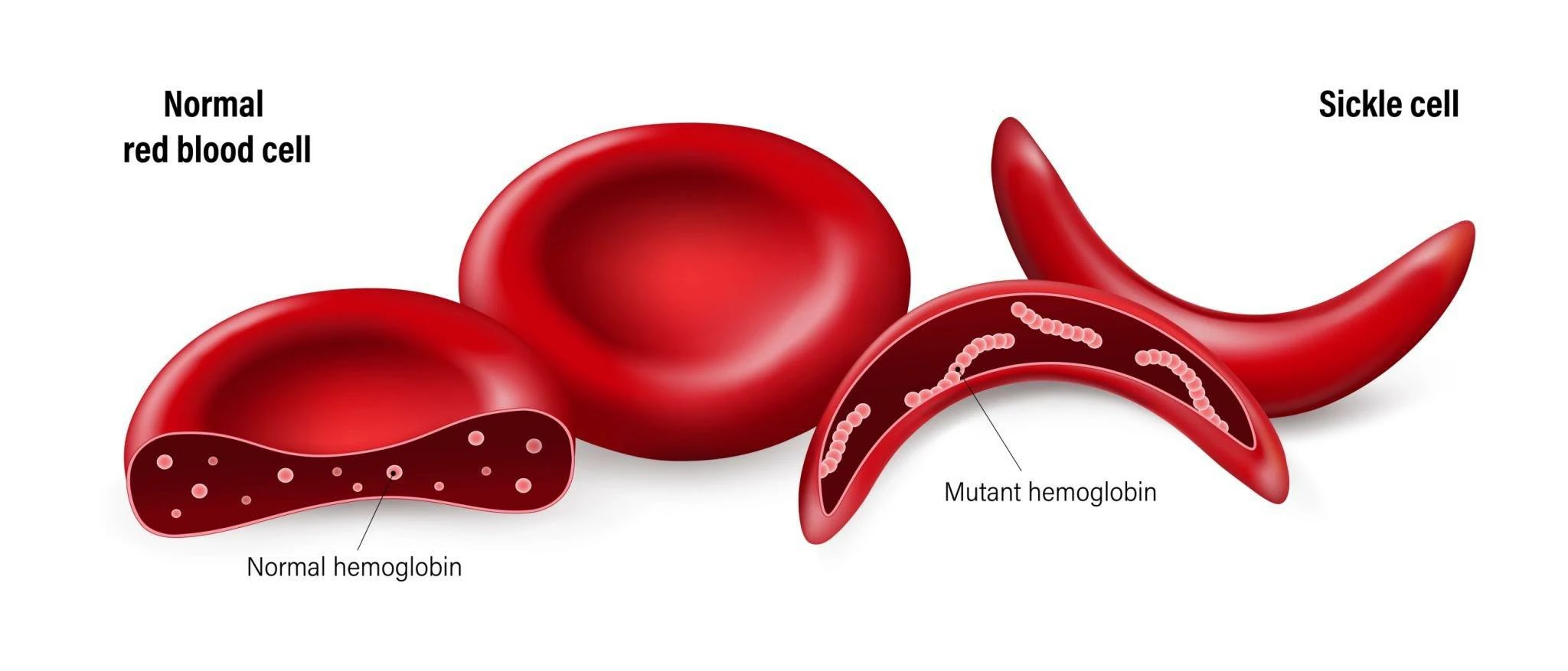
Sickle Cell Disease
This condition arises when the body generates an atypical form of hemoglobin known as hemoglobin S, as a result of a mutation in the beta-globin gene HBB.
Thalassemia
This is a hereditary disorder marked by a reduction in the body's hemoglobin production, which occurs due to a diminished or complete absence of one or more globin subunits.
Polycythemia
It is distinguished by elevated levels of hemoglobin in the bloodstream.
Methemoglobinemia
This condition is defined by a diminished capacity of hemoglobin to transport oxygen, resulting from the alteration of iron from its reduced Fe+2 (ferrous) state to the oxidized Fe+3 (ferric) state.
Hemoglobinuria
The detection of hemoglobin in the urine.
Hereditary persistence of fetal hemoglobin (HPFH)
This is a non-threatening condition marked by the significant presence of fetal hemoglobin, known as hemoglobin F, in adults.



~1.webp)

.webp)

