Table of Contents
- What is Most Probable Number (MPN)
- Presumptive Test of MPN Test
- Confirmed Test of MPN Test
- Completed Test of MPN Test
- Objectives of MPN Test
- Procedure of MPN Test
- Results of MPN Test
- Completed Test
- Uses of MPN Test
- Advantages of MPN Test
- Limitations of MPN Test
What is Most Probable Number (MPN)
- The Most Probable Number (MPN) test is a statistical method that estimates the concentration of microorganisms in a sample based on their random distribution per unit volume.
- In this technique, specific volumes of water are introduced into a series of tubes containing a liquid indicator growth medium.
- Tubes that receive one or more indicator bacteria exhibit growth and a characteristic color change, while tubes containing only the water sample without indicator bacteria show no color change.
- The number and distribution of positive (color change) and negative (no color change) reactions are used to estimate the MPN of indicator organisms in the sample by referring to statistical tables.
- The MPN test is conducted in three stages:
- Presumptive test
- Confirmed test
- Completed test
Presumptive Test of MPN Test
This test is a specific enrichment procedure for detecting coliform bacteria. It is conducted in fermentation tubes filled with a selective growth medium, typically MacConkey lactose broth, which contains inverted Durham tubes to detect fermentation gas. A series of lactose broth tubes are inoculated with measured amounts of the water sample to be tested. The series may consist of three or four groups, each containing three, five, or more tubes.
The main selective factors in the medium are lactose, sometimes a surfactant such as Na-lauryl sulfate or Na-taurocholate (bile salt), and often a pH indicator dye, such as bromcresol purple or brilliant green, to facilitate the detection of acid production. Lactose acts selectively because many bacteria cannot ferment this sugar, whereas coliform bacteria and several other bacterial types can. The surfactant and dye do not inhibit coliform bacteria but do inhibit many other bacteria, such as spore formers.
Confirmed Test of MPN Test
This test is conducted to confirm the presence of coliform bacteria when a positive or doubtful result is obtained from the presumptive test.
A loopful of growth from a presumptive positive tube is transferred into a tube of brilliant green lactose bile (BGLB) 2% broth (or another lactose broth) and incubated at 35°C for 48 hours. This selective medium is used for detecting coliform bacteria in water, dairy, and other food products. Lactose serves as the selective agent, and the broth tube contains a Durham tube to detect gas production.
Additionally, a plate of LES Endo agar (or EMB agar) is streaked with a loopful of growth from a positive tube and incubated at 35°C for 18–24 hours. Typical coliform bacteria, such as E. coli and Enterobacter aerogenes, show good growth on this medium, forming red to black colonies with dark centers or a sheen. Salmonella typhi also exhibits good growth but produces colorless colonies, while S. aureus growth is inhibited altogether.
Completed Test of MPN Test
This test is used to further confirm doubtful results and, if desired, to verify positive confirmed test results. A typical coliform colony from an LES Endo agar plate is inoculated into a tube of brilliant green bile broth and onto the surface of a nutrient agar slant. These are then incubated at 35°C for 24 hours. After incubation, the broth is examined for gas production, and a Gram stain is performed on the organisms from the nutrient agar slant. If the organism is a Gram-negative, non-spore-forming rod that produces gas in the lactose tube, it confirms the presence of coliforms in the water sample.
Objectives of MPN Test
- To quantify the number of bacteria present in drinking water using the MPN method.
- To identify the types of bacteria present in the drinking water sample.
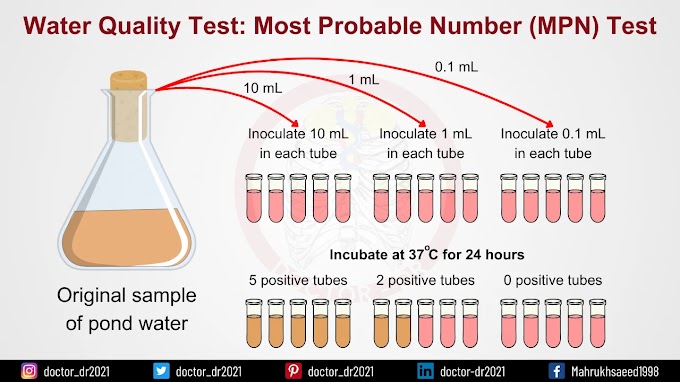
Procedure of MPN Test
I. Presumptive Test
- Prepare MacConkey purple media in both single and double strength in test tubes, each containing a Durham tube. Autoclave the prepared media.
- Arrange three sets of test tubes, each containing five tubes: one set with 10 ml of double strength (DS) media and the other two sets with 10 ml of single strength (SS) media.
- Using sterile pipettes, transfer 10 ml of the water sample into each of the DS broth tubes.
- Transfer 1 ml of the water sample into each of the five tubes in one set of SS broth.
- Transfer 0.1 ml of the water sample into each of the five tubes in the remaining set of SS broth.
- Incubate the tubes at 37°C for 24 hours.
- After incubation, check for gas production in the Durham tubes and observe any color change in the media.
- Record the number of positive results from each set and compare the results with the standard chart to determine the presumptive coliform count per 100 ml of the water sample.
II. Confirmed Test
Some microorganisms other than coliforms can also produce acid and gas from lactose fermentation. To confirm the presence of coliforms, a confirmatory test is performed. In this test, a loopful of suspension from a positive tube is inoculated into a 3 ml lactose broth or brilliant green lactose fermentation tube, as well as onto an agar plate (EMB agar or Endo Agar) or slant.
A. Inoculation of the lactose-broth
- Incubate the inoculated lactose-broth fermentation tubes at 37°C.
- Inspect for gas formation after 24 ± 2 hours.
- If no gas production is observed, continue incubation for up to 48 ± 3 hours to check for gas production.
B. Inoculation in media slants
- Take a loopful of suspension from a positive tube and inoculate it onto the surface of the agar slants.
- Incubate the agar slants at 37°C for 24 ± 2 hours.
- Examine the colonies macroscopically.
III. Completed Test
- Transfer a typical coliform colony from the agar plate into a tube of brilliant green bile broth containing a Durham tube and onto the surface of a nutrient agar slant.
- Incubate the tubes at 35°C for 24 hours.
- After incubation, check the broth for gas production and perform Gram staining on the organisms from the nutrient agar slant.
Results of MPN Test
A. Presumptive Test
- Positive: A positive result is indicated by the formation of 10% or more gas in the Durham tube within 24 to 48 hours, along with turbidity in the growth medium and a color change in the medium. This suggests the presence of coliform bacteria and potentially indicates fecal pollution.
- Negative: A negative result is characterized by no growth or gas formation in the Durham tube.
B. Confirmed Test
- Positive: The presence of gas in the lactose broth and the identification of coliform-like colonies on EMB agar indicate that a coliform member is present in the sample. Coliforms typically form colonies with a greenish metallic sheen on EMB agar, distinguishing them from non-coliform colonies, which do not exhibit this sheen. The presence of typical colonies at high temperatures (44.5 ± 0.2°C) suggests the presence of thermotolerant E. coli.
- Negative: A negative result is indicated by the absence of gas formation in the lactose broth or the failure to observe coliform-like colonies on the EMB agar.
Completed Test
- Positive: A positive result is indicated by the presence of gas in the brilliant green bile broth and the observation of Gram-negative, non-spore-forming rods on the nutrient agar slant. This confirms the presence of coliform bacteria and suggests potential contamination of the water sample with fecal matter.
- Negative: A negative result is characterized by the absence of growth and gas formation in the broth and the lack of Gram-negative, non-spore-forming rods upon Gram staining.
Uses of MPN Test
- The MPN test is widely used to estimate microbial populations in soils, waters, and agricultural products.
- It is especially valuable for samples containing particulate material that may disrupt plate count enumeration methods.
- The test is also considered as an alternative method for environmental monitoring studies.
- Additionally, it is useful for counting bacteria that do not readily form colonies on agar plates or membrane filters but grow efficiently in liquid media.
Advantages of MPN Test
- Ease of Interpretation: The MPN test is straightforward to interpret, whether by visual observation or gas emission.
- Sample Toxins: The test effectively dilutes sample toxins, reducing their impact on results.
- Handling Turbid Samples: It is an effective method for analyzing highly turbid samples, such as sediments, sludge, and mud.
- Versatility: The test allows for the analysis of samples that cannot be processed by membrane filtration methods.
Limitations of MPN Test
- The MPN test often has lower accuracy and precision compared to other methods, making it a last resort when alternative counting methods are unsuitable.
- The test can be laborious and costly due to the need for materials, glassware, and incubator space.
- It has a relatively large margin of error, which can affect the reliability of results.